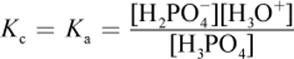MCAT General Chemistry Review
Chapter 6: Equilibrium
Conclusion
We’ve discussed some very important concepts and principles in the past two chapters related to the studies of reaction rates and chemical equilibria. In this chapter, we began with the law of mass action and the significance of the equilibrium state of a chemical reaction. With our understanding of the significance of Keq and Q, we are able to predict the direction that a reaction will go in response to various stresses—concentration, pressure, or temperature changes—that might be applied to a system.
The concept of homeostasis in biology is a direct result of the energy associated with disturbing equilibria in the body. Reactions are often held slightly out of the equilibrium state to generate energy. Many pathologies you will encounter in your future career in medicine will have a fundamental basis in disturbed chemical equilibria—just wait until you start ordering metabolic panels on your patients!
Concept Summary
Equilibrium
· Reversible reactions eventually reach a state in which energy is minimized and entropy is maximized.
o Chemical equilibria are dynamic—the reactions are still occurring, just at a constant rate.
o The concentrations of reactants and products remain constant because the rate of the forward reaction equals the rate of the reverse reaction.
· The law of mass action gives the expression for the equilibrium constant, Keq. The reaction quotient, Q, has the same form but can be calculated at any concentrations of reactants and products.
o Q is a calculated value that relates the reactant and product concentrations at any given time during a reaction.
o Keq is the ratio of products to reactants at equilibrium, with each species raised to its stoichiometric coefficient. Keq for a reaction is constant at a constant temperature.
o Pure solids and liquids do not appear in the law of mass action; only gases and aqueous species do.
· Comparison of Q to Keq provides information about where the reaction is with respect to its equilibrium state.
o If Q < Keq, ΔG < 0, and the reaction proceeds in the forward direction.
o If Q = Keq, ΔG = 0, and the reaction is in dynamic equilibrium.
o If Q > Keq, ΔG > 0, and the reaction proceeds in the reverse direction.
Le Châtelier’s Principle
· Le Châtelier’s principle states that when a chemical system experiences a stress, it will react so as to restore equilibrium.
· There are three main types of stresses applied to a system: changes in concentration, pressure and volume, and temperature.
o Increasing the concentration of reactants or decreasing the concentration of products will shift the reaction to the right. Increasing the concentration of products or decreasing the concentration of reactants will shift the reaction to the left.
o Increasing pressure on a gaseous system (decreasing its volume) will shift the reaction toward the side with fewer moles of gas. Decreasing pressure on a gaseous system (increasing its volume) will shift the reaction toward the side with more moles of gas.
o Increasing the temperature of an endothermic reaction or decreasing the temperature of an exothermic reaction will shift the reaction to the right. Decreasing the temperature of an endothermic reaction or increasing the temperature of an exothermic reaction will shift the reaction to the left.
Kinetic and Thermodynamic Control
· Reactions may have both kinetic and thermodynamic products that can be regulated by temperature and the presence of a catalyst.
o Kinetic products are higher in free energy than thermodynamic products and can form at lower temperatures. These are sometimes termed “fast” products because they can form more quickly under such conditions.
o Thermodynamic products are lower in free energy than kinetic products and are therefore more stable. Despite proceeding more slowly than the kinetic pathway, the thermodynamic pathway is more spontaneous (more negative ΔG).
Answers to Concept Checks
· 6.1
1.
|
Keq |
Direction of Reaction |
ΔG |
|
5.0 × 10−2 |
At equilibrium: no net reaction |
0 |
|
5.0 × 10−3 |
Qc > Keq: proceeds toward reactants (left) |
Positive |
|
0.25 × 10−1 |
Qc < Keq: proceeds toward products (right) |
Negative |
2.
|
⋅ |
|
|
⋅ |
|
· 6.2
1.
· Increasing pH of H2SO4 (aq) ⇌ H+ (aq) + HSO4− (aq): [H+] decreases, shifting reaction to the right.
· Decreasing pressure of 2 C (s) + O2 (g) ⇌ 2 CO (g): Reaction shifts right, favoring the side with more moles of gas.
· Warming CH4 (g) + 2O2 (g) ⇌ CO2 (g) + 2 H2O (l) + heat: Reaction shifts left, using the additional heat energy to produce more reactants.
· Removing water from H3PO4 (aq) + H2O (l) ⇌ H3O+ (aq) + H2PO4− (aq): Reaction shifts left. All concentrations would increase proportionately; because there are more products than reactants (and the stoichiometric coefficient is 1 for each reactant and product), the value of Q will increase.
· 6.3
1. Kinetic products are favored at low temperatures with low heat transfer. Thermodynamic products are favored at high temperatures with high heat transfer.
2. Kinetic pathways require a smaller gain in free energy to reach the transition state. They also have a higher free energy of the products, with a smaller difference in free energy between the transition state and the products.
Equations to Remember
(6.1) Equilibrium constant: 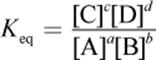
(6.2) Reaction quotient: 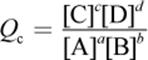
Shared Concepts
· Biochemistry Chapter 2
o Enzymes
· General Chemistry Chapter 5
o Chemical Kinetics
· General Chemistry Chapter 7
o Thermochemistry
· General Chemistry Chapter 9
o Solutions
· General Chemistry Chapter 10
o Acids and Bases
· Organic Chemistry Chapter 2
o Isomers