MCAT General Chemistry Review
Chapter 7: Thermochemistry
7.1 Systems and Processes
Students often have some anxiety over what constitutes a system and what—by exclusion from the system—constitutes the surroundings or environment. Perhaps the problem isn’t so much the definitions themselves but the way in which the boundary between the two can be shifted to suit the needs of the experimenter or observer. Simply put, the system is the matter that is being observed—the total amount of reactants and products in a chemical reaction. It could be the amount of solute and solvent used to create a solution. It could be the gas inside a balloon. Then, thesurroundings, or environment, are everything outside of that system. However, the boundary between system and surroundings is not permanently fixed, and can be moved. For example, one might consider the mass of coffee in a coffee cup to be the system and the cup containing it to be the environment. This setup would likely be used if someone was interested in determining the amount of heat transferred from the hot coffee to the cooler coffee cup. Alternatively, one might define the system as the hot coffee and the cup together, and the environment as the air surrounding the coffee cup. This setup would likely be used if someone was interested in calculating the heat exchange between the hot coffee and cup system and the cooler surrounding air. The boundary can be extended out farther and farther, until the entire mass of the universe is ultimately included in the system. At this point, there are no surroundings. Again, where the boundary is placed is a decision based on what phenomenon one is interested in studying.
THERMODYNAMIC TERMINOLOGY
Systems can be characterized by whether or not they can exchange heat or matter with the surroundings. A system may be characterized as follows:
· Isolated: The system cannot exchange energy (heat and work) or matter with the surroundings; for example, an insulated bomb calorimeter.
· Closed: The system can exchange energy (heat and work) but not matter with the surroundings; for example, a steam radiator.
· Open: The system can exchange both energy (heat and work) and matter with the surroundings; for example, a pot of boiling water.
When a system experiences a change in one or more of its properties (such as concentrations of reactants or products, temperature, or pressure), it undergoes a process.
While processes, by definition, are associated with a change of the state of a system, some processes are uniquely identified by some property that is constant throughout the process. Many of these processes create special conditions because they allow us to simplify the first law of thermodynamics:
ΔU = Q – W
Equation 7.1
where ΔU is the change in internal energy of the system, Q is the heat added to the system, and W is the work done by the system.
For example, isothermal processes occur when the system’s temperature is constant. Constant temperature implies that the total internal energy of the system (U) is constant throughout the process. This is because temperature and internal energy are directly proportional. When U is constant,ΔU = 0 and the first law simplifies to Q = W (the heat added to the system equals the work done by the system). An isothermal process appears as a hyperbolic curve on a pressure–volume graph (P–V graph). Work is represented by the area under such a curve, as shown in Figure 7.1.
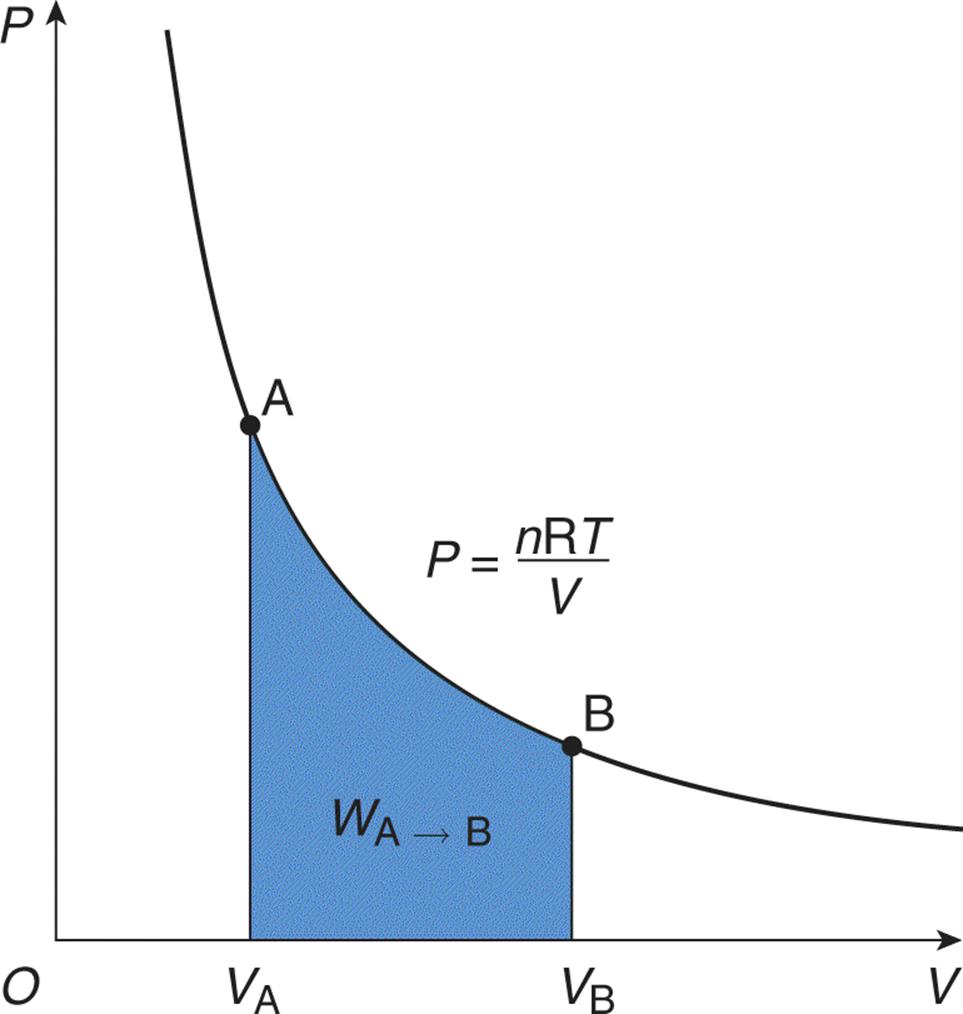 Figure 7.1. Graph of an Isothermal Expansion Temperature is constant in an isothermal process; thus, the area under the curve represents not only the work performed by the gas, but also the heat that entered the system.
Figure 7.1. Graph of an Isothermal Expansion Temperature is constant in an isothermal process; thus, the area under the curve represents not only the work performed by the gas, but also the heat that entered the system.
Adiabatic processes occur when no heat is exchanged between the system and the environment; thus, the thermal energy of the system is constant throughout the process. When Q = 0, the first law simplifies to ΔU = –W (the change in internal energy of the system is equal to work done onthe system [the opposite of work done by the system]). An adiabatic process also appears hyperbolic on a P–V graph, as shown in Figure 7.2.
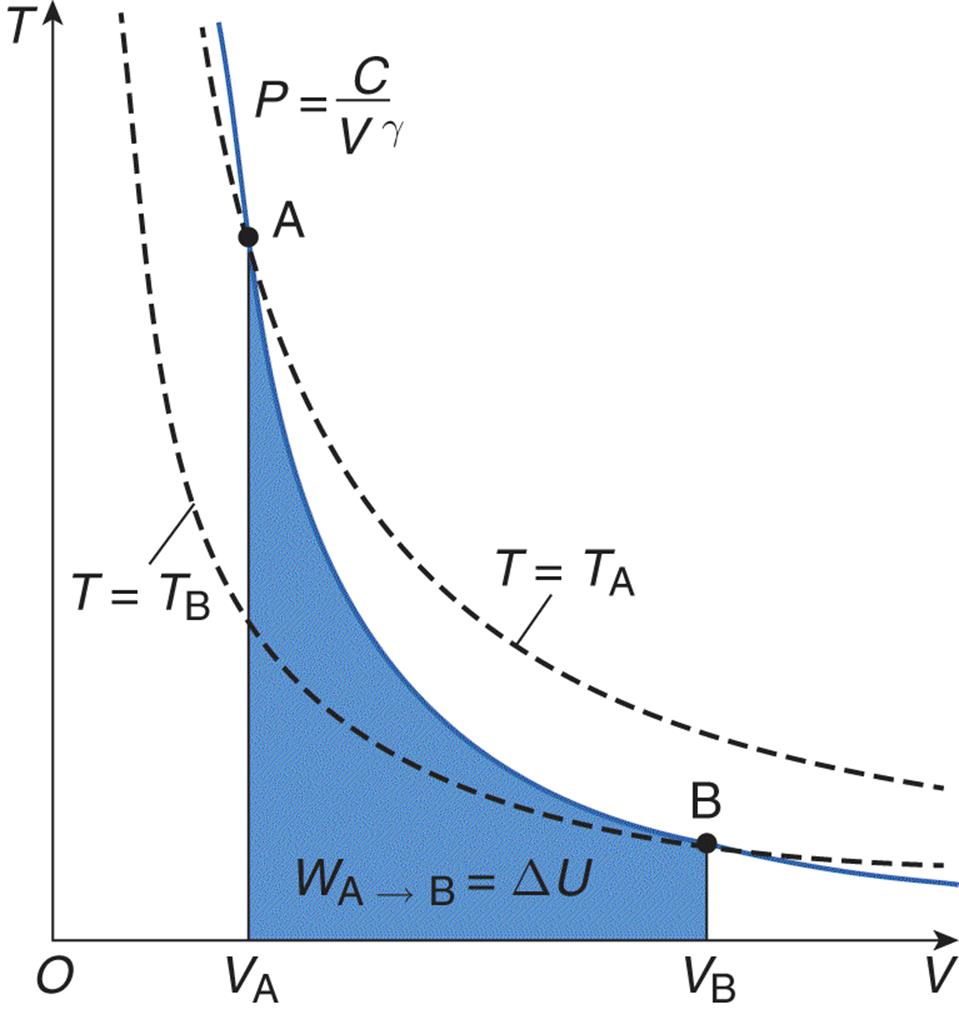 Figure 7.2. Graph of an Adiabatic Expansion Heat exhange is zero in an adiabatic process; temperature is not constant (as shown by the dotted lines).
Figure 7.2. Graph of an Adiabatic Expansion Heat exhange is zero in an adiabatic process; temperature is not constant (as shown by the dotted lines).
Isobaric processes occur when the pressure of the system is constant. Isothermal and isobaric processes are common because it is usually easy to control temperature and pressure. Isobaric processes do not alter the first law, but note that an isobaric process appears as a flat line on a P–V graph, as shown in Figure 7.3.
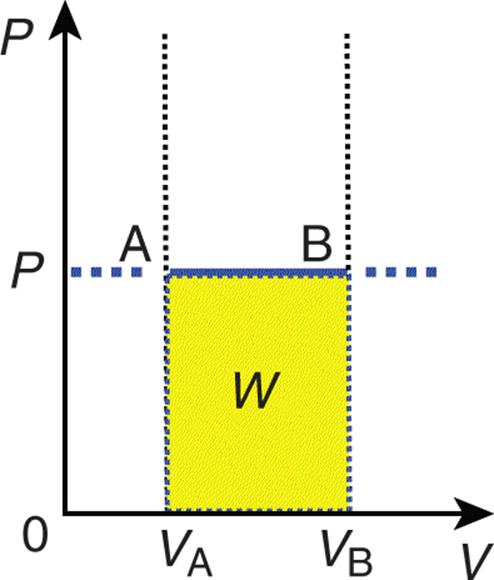 Figure 7.3. Graph of an Isobaric Expansion Pressure is constant in an isobaric process; the slope of the line is therefore zero.
Figure 7.3. Graph of an Isobaric Expansion Pressure is constant in an isobaric process; the slope of the line is therefore zero.
Finally, isovolumetric (isochoric) processes experience no change in volume. Because the gas neither expands nor compresses, no work is performed in such a process. Thus, the first law simplifies to ΔU = Q (the change in internal energy is equal to the heat added to the system). An isovolumetric process is a vertical line on a P–V graph; the area under the curve, which represents the work done by the gas, is zero.
BRIDGE
The terms isothermal, adiabatic, isobaric, and isovolumetric (isochoric) may seem familiar because they are also discussed in Chapter 3 of MCAT Physics and Math Review.
Processes themselves can also be classified as spontaneous or nonspontaneous. A spontaneous process is one that can occur by itself without having to be driven by energy from an outside source. Calculating the change in the Gibbs free energy (ΔG) for a process, such as a chemical reaction, allows us to predict whether the process will be spontaneous or nonspontaneous. As discussed later in the chapter, the same quantities that are used to calculate the change in the Gibbs free energy, ΔH and ΔS, can also tell us whether the process will be temperature dependent; that is, spontaneous at some temperatures and nonspontaneous at others.
Spontaneous reactions, as mentioned in Chapters 5 and 6 of MCAT General Chemistry Review, will not necessarily happen quickly and may not go to completion. Many spontaneous reactions have very high activation energies and, therefore, rarely take place. For example, when was the last time you saw a match ignite itself spontaneously? However, providing a quantity of thermal energy (generated by the friction associated with striking the match) that equals or exceeds the activation energy will allow the match to light and burn spontaneously. At this point, the combustion of the chemical components of the match using molecular oxygen in the air will not need any additional external energy once the activation energy has been supplied.
Some spontaneous reactions proceed very slowly. The role of enzymes—biological catalysts—is to selectively enhance the rate of certain spontaneous (but slow) chemical reactions so that the biologically necessary products can be formed at a rate sufficient for sustaining life. As we discussed in Chapter 6 of MCAT General Chemistry Review, some reactions do not go to completion but settle into a low-energy state called equilibrium. Spontaneous reactions may go to completion, but many simply reach equilibrium with dynamically stable concentrations of reactants and products. A common method for supplying energy for nonspontaneous reactions is by coupling nonspontaneous reactions to spontaneous ones, as shown in Figure 7.4.
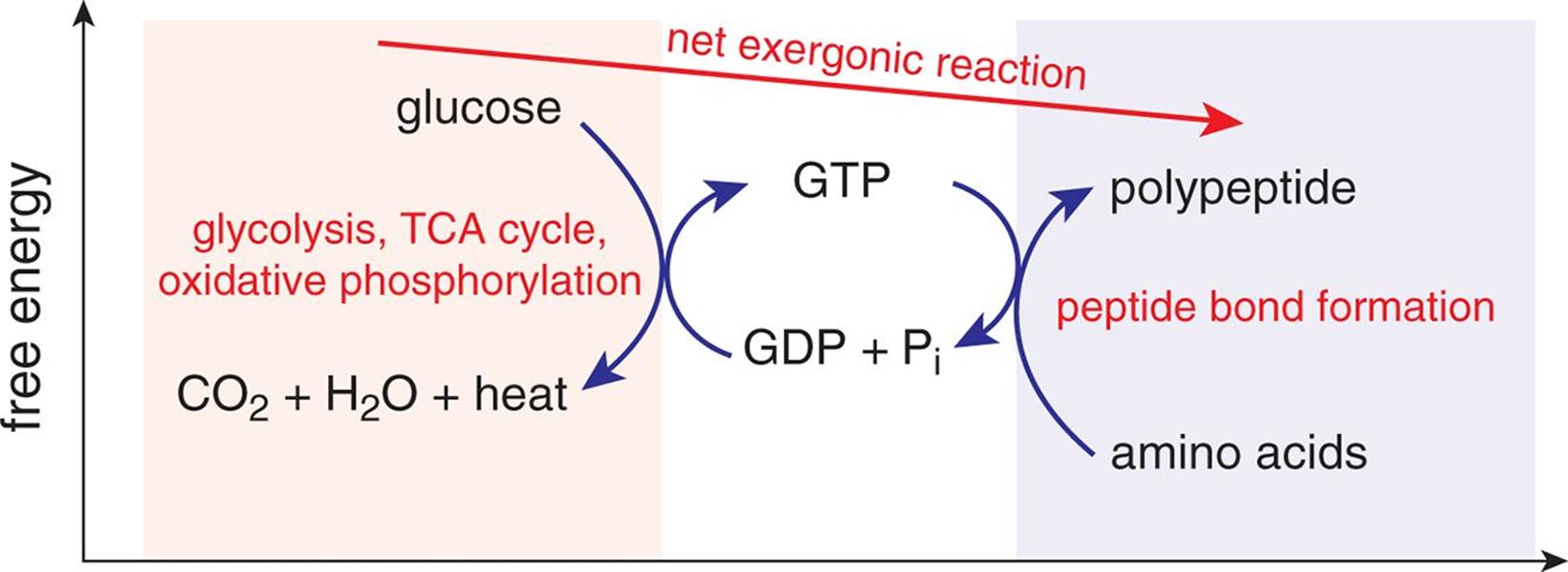 Figure 7.4. Coupling of Reactions The combustion of glucose is exergonic; the formation of peptide bonds is endergonic. Energy from the combustion of glucose can be stored in the bonds in GTP, which are then lysed to provide the energy for forming peptide bonds.
Figure 7.4. Coupling of Reactions The combustion of glucose is exergonic; the formation of peptide bonds is endergonic. Energy from the combustion of glucose can be stored in the bonds in GTP, which are then lysed to provide the energy for forming peptide bonds.
MCAT Concept Check 7.1:
Before you move on, assess your understanding of the material with these questions.
1. A person snaps an ice pack and places it on his or her leg. In terms of energy transfer, what would be considered the system and what would be the surroundings in this scenario?
· System:
· Surroundings:
2. What is unique about each of the following types of processes?
· Isothermal:
· Adiabatic:
· Isobaric:
· Isovolumetric (isochoric):