MCAT General Chemistry Review
Chapter 7: Thermochemistry
7.2 States and State Functions
The state of a system can be described by certain macroscopic properties. These properties, or state functions, describe the system in an equilibrium state. They cannot describe the process of the system; that is, how the system got to its current equilibrium. They are useful only for comparing one equilibrium state to another. The pathway taken from one equilibrium state to another is described quantitatively by the process functions, the most important of which are work (W) and heat (Q).
OVERVIEW
The state functions include pressure (P), density (ρ), temperature (T), volume (V), enthalpy (H), internal energy (U), Gibbs free energy (G), and entropy (S). When the state of a system changes from one equilibrium to another, one or more of these state functions will change. In addition, while state functions are independent of the path (process) taken, they are not necessarily independent of one another. For example, Gibbs free energy is related to enthalpy, temperature, and entropy.
MNEMONIC
State functions: When I’m under pressure and feeling dense, all I want to do is watch TV and get HUGS.
Pressure (P), density (ρ), temperature (T), volume (V), enthalpy (H), internal energy (U), Gibbs free energy (G), and entropy (S).
Because systems can be in different equilibrium states at different temperatures and pressures, a set of standard conditions has been defined for measuring the enthalpy, entropy, and Gibbs free energy changes of a reaction. The standard conditions are defined as 25°C (298 K), 1 atm pressure, and 1 M concentrations. Don’t confuse standard conditions with standard temperature and pressure (STP), for which the temperature is 0°C (273 K) and pressure is 1 atm. Standard conditions are used for kinetics, equilibrium, and thermodynamics problems; STP is used for ideal gas calculations.
MCAT EXPERTISE
On the MCAT, be sure that you do not confuse standard conditions in thermodynamics with standard temperature and pressure (STP), which is used in gas law calculations:
· Standard conditions: 25°C (298 K), 1 atm pressure, 1 M concentrations
· STP: 0°C (273 K), 1 atm pressure
Under standard conditions, the most stable form of a substance is called the standard state of that substance. You should recognize the standard states for some elements and compounds commonly encountered on the MCAT. For example, H2 (g), H2O (l), NaCl (s), O2 (g), and C (s,graphite) are the most stable forms of these substances under standard conditions. Recognizing whether or not a substance is in its standard state is important for thermochemical calculations, such as heats of reactions and—in particular—heats of formation. The changes in enthalpy, entropy, and free energy that occur when a reaction takes place under standard conditions are called the standard enthalpy, standard entropy, and standard free energy changes, respectively, and are symbolized by ΔH°, ΔS°, and ΔG°. The degree sign in these variables represents zero, as the standard state is used as the “zero point” for all thermodynamic calculations.
PHASE CHANGES
Phase diagrams are graphs that show the standard and nonstandard states of matter for a given substance in an isolated system, as determined by temperatures and pressures. Phase changes (solid ⇋ liquid ⇋ gas) are reversible, and an equilibrium of phases will eventually be reached at any given combination of temperature and pressure. For example, at 0°C and 1 atm in an isolated system, ice and water exist in an equilibrium. In other words, some of the ice may absorb heat (from the liquid water) and melt, but because that heat is being removed from the liquid water, an equal amount of the liquid water will freeze and form ice. Thus, the relative amounts of ice and water remain constant. Equilibrium between the liquid and gas states of water will be established in a closed container at room temperature and atmospheric pressure, such as a plastic water bottle with the cap screwed on tightly. Most of the water in the bottle will be in the liquid phase, but a small number of molecules at the surface will gain enough kinetic energy to escape into the gas phase; likewise, a small number of gas molecules will lose sufficient kinetic energy to reenter the liquid phase. After a while, equilibrium is established, and the relative amounts of water in the liquid and gas phases become constant—at standard conditions, equilibrium occurs when the air above the water has about 3 percent water vapor by mass. Phase equilibria are analogous to the dynamic equilibria of reversible chemical reactions: the concentrations of reactants and products are constant because the rates of the forward and reverse reactions are equal.
KEY CONCEPT
As with all equilibria, the rates of the forward and reverse processes will be the same when considering phase changes.
Gas–Liquid Equilibrium
The temperature of any substance in any phase is related to the average kinetic energy of the molecules that make up the substance. However, not all of the molecules have exactly the same instantaneous speeds. Therefore, the molecules possess a range of instantaneous kinetic energy values. In the liquid phase, the molecules are relatively free to move around one another. Some of the molecules near the surface of the liquid may have enough kinetic energy to leave the liquid phase and escape into the gaseous phase. This process is known as evaporation or vaporization. Each time the liquid loses a high-energy particle, the temperature of the remaining liquid decreases. Evaporation is an endothermic process for which the heat source is the liquid water. Of course, the liquid water itself may be receiving thermal energy from some other source, as in the case of a puddle of water drying up under the hot summer sun or a pot of water on the stovetop. Given enough energy, the liquid will completely evaporate.
Boiling is a specific type of vaporization that occurs only under certain conditions. Any liquid will lose some particles to the vapor phase over time; however, boiling is the rapid bubbling of the entire solution with rapid release of the liquid as gas particles. While evaporation happens in all liquids at all temperatures, boiling can only occur above the boiling point of a liquid and involves vaporization through the entire volume of the liquid.
In a covered or closed container, the escaping molecules are trapped above the solution. These molecules exert a countering pressure, which forces some of the gas back into the liquid phase; this process is called condensation. Condensation is facilitated by lower temperature or higher pressure. Atmospheric pressure acts on a liquid in a manner similar to that of an actual physical lid. As evaporation and condensation proceed, the respective rates of the two processes become equal, and equilibrium is reached. The pressure that the gas exerts over the liquid at equilibrium is the vapor pressure of the liquid. Vapor pressure increases as temperature increases because more molecules have sufficient kinetic energy to escape into the gas phase. The temperature at which the vapor pressure of the liquid equals the ambient (also known as external, applied, incident, or atmospheric) pressure is called the boiling point.
Liquid–Solid Equilibrium
We’ve already illustrated the equilibrium that can exist between the liquid and the solid phases of water at 0°C. Even though the atoms or molecules of a solid are confined to specific locations, each atom or molecule can undergo motions about some equilibrium position. These vibrational motions increase when heat is applied. From our understanding of entropy, we can say that the availability of energy microstates increases as the temperature of the solid increases. In basic terms, this means that the molecules have greater freedom of movement, and energy disperses. If atoms or molecules in the solid phase absorb enough energy, the three-dimensional structure of the solid will break down, and the atoms or molecules will escape into the liquid phase. The transition from solid to liquid is called fusion or melting. The reverse process, from liquid to solid, is called solidification, crystallization, or freezing. The temperature at which these processes occur is called the melting point or freezing point, depending on the direction of the transition. Whereas pure crystalline solids have distinct, very precise melting points, amorphous solids, such as glass, plastic, and candle wax, tend to melt (or solidify) over a larger range of temperatures due to their less-ordered molecular structure.
Gas–Solid Equilibrium
The final phase equilibrium is that which exists between the gaseous and solid phases. When a solid goes directly into the gas phase, the process is called sublimation. Dry ice (solid CO2) sublimes at room temperature and atmospheric pressure; the absence of the liquid phase makes it a convenient dry refrigerant. The reverse transition, from the gaseous to the solid phase, is called deposition. In organic chemistry laboratories, a device known as a cold finger may be used to purify a product that is heated under reduced pressure, causing it to sublimate. The desired product is usually more volatile than the impurities, so the gas is purer than the original product and the impurities are left in the solid state. The gas then deposits onto the cold finger, which has cold water flowing through it, yielding a purified solid product that can be collected.
PHASE DIAGRAMS
Phase diagrams are graphs that show the temperatures and pressures at which a substance will be thermodynamically stable in a particular phase. They also show the temperatures and pressures at which phases will be in equilibrium.
The lines on a phase diagram are called the lines of equilibrium or the phase boundaries and indicate the temperature and pressure values for the equilibria between phases. The lines of equilibrium divide the diagram into three regions corresponding to the three phases—solid, liquid, and gas—and they themselves represent the phase transformations. The phase diagram for a single compound is shown in Figure 7.5.
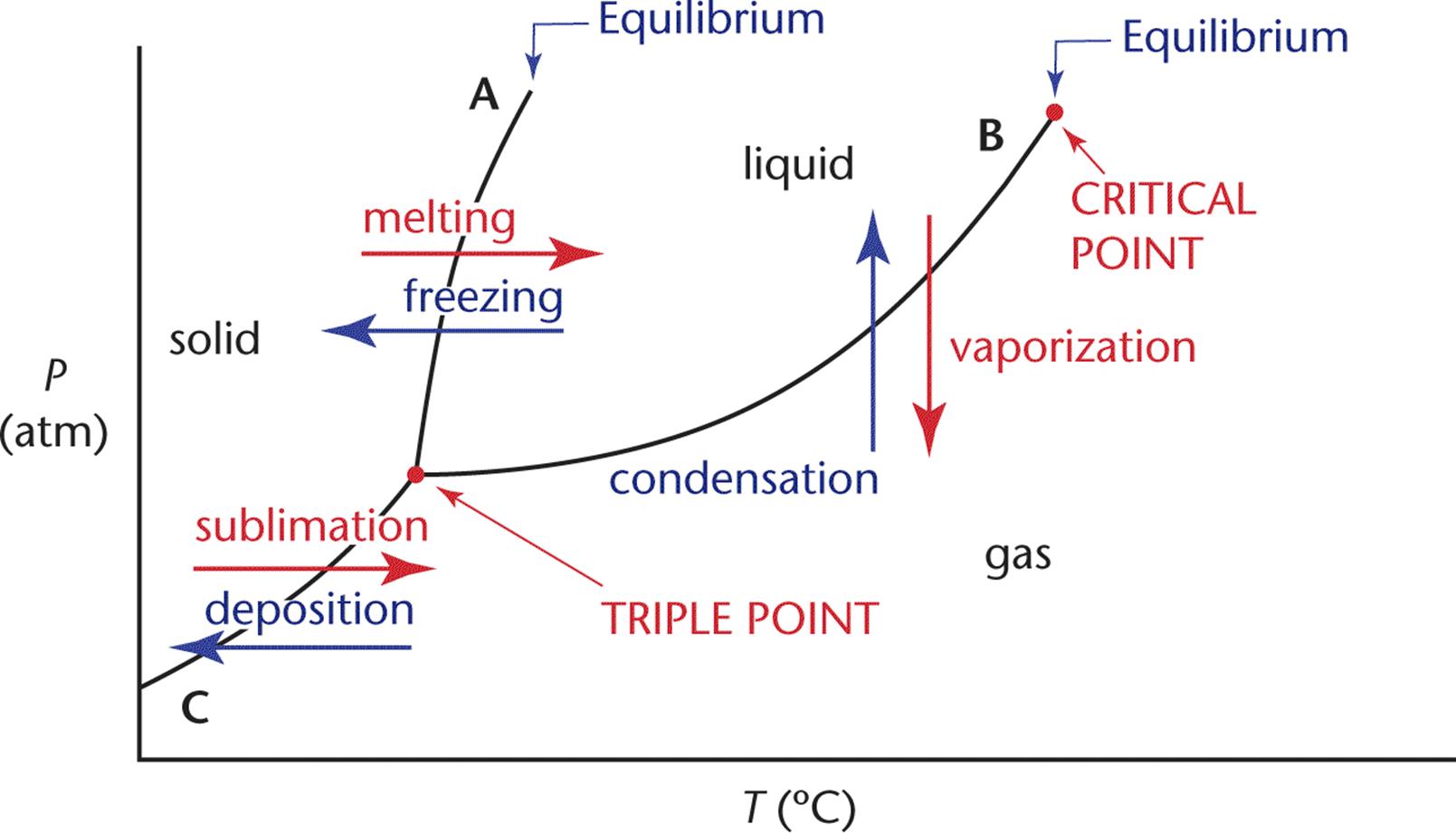 Figure 7.5. Phase Diagram for a Single Compound
Figure 7.5. Phase Diagram for a Single Compound
MCAT EXPERTISE
On the MCAT, you should be able to identify and understand each area and every line of a phase diagram.
REAL WORLD
Because of water’s unique properties, ice floats and skates flow smoothly over ice rinks. This all “boils” down to the negative slope of the solid–liquid equilibrium line in its phase diagram. Because the density of ice is less than that of liquid water, an increase in pressure (at a constant temperature) will actually melt ice (the opposite of what is seen for the substance in Figure 7.5).
Line A represents the solid–liquid interface, line B the liquid–gas interface, and line C the solid–gas interface. In general, the gas phase is found at high temperatures and low pressures, the solid phase is found at low temperatures and high pressures, and the liquid phase is found at moderate temperatures and moderate pressures. The point at which the three phase boundaries meet is called the triple point. This is the temperature and pressure at which the three phases exist in equilibrium. The phase boundary that separates the solid and the liquid phases extends indefinitely from the triple point. The phase boundary between the liquid and gas phases, however, terminates at a point called the critical point. This is the temperature and pressure above which there is no distinction between the phases. Although this may seem to be an impossibility—after all, it’s always possible to distinguish between the liquid and the solid phase—such supercritical fluids are perfectly logical. As a liquid is heated in a closed system its density decreases and the density of the vapor sitting above it increases. The critical point is the temperature and pressure at which the two densities become equal and there is no distinction between the two phases. The heat of vaporization at this point and for all temperatures and pressures above the critical point values is zero.
MCAT Concept Check 7.2:
Before you move on, assess your understanding of the material with these questions.
1. What are standard conditions? When are standard conditions used for calculations?
2. What is the definition of a state function? A process function?
· State function:
· Process function:
3. List at least five common state functions:
·
·
·
·
·
4. What is the definition of the triple point? The critical point?
· Triple point:
· Critical point: