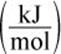MCAT General Chemistry Review
Chapter 7: Thermochemistry
Practice Questions
1. Consider the cooling of an ideal gas in a closed system. This process is illustrated in the pressure–volume graph shown in the following figure.
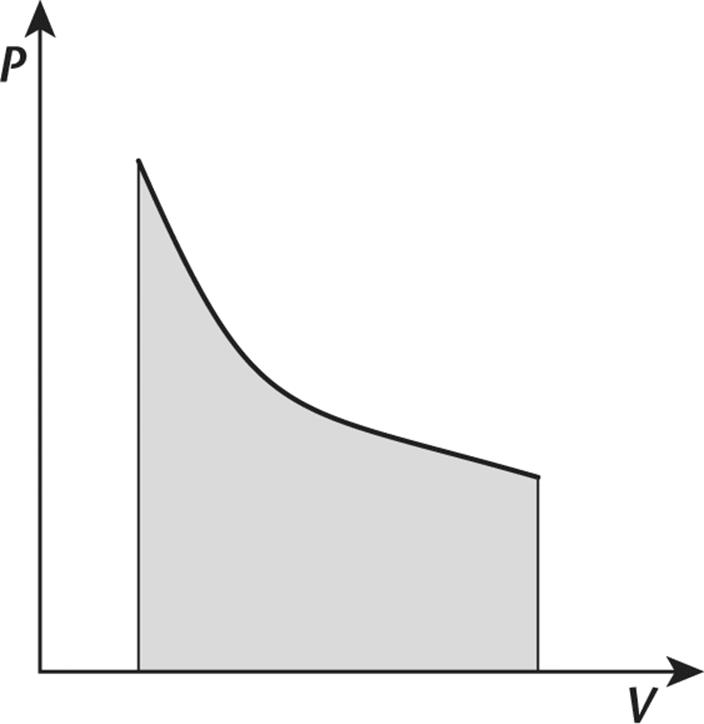
Based on this information, the process may be:
1. adiabatic.
2. isobaric.
3. isothermal.
4. isochoric.
2. A reaction has a positive entropy and enthalpy. What can be inferred about the progress of this reaction from this information?
1. The reaction is spontaneous.
2. The reaction is nonspontaneous.
3. The reaction is at equilibrium.
4. There is not enough information to determine whether the reaction is spontaneous or not.
3. Pure sodium metal spontaneously combusts upon contact with room temperature water. What is true about the equilibrium constant of this combustion reaction at 25°C?
1. Keq < 0
2. 0 < Keq < 1
3. Keq = 1
4. Keq > 1
4. Which of the following processes has the most exothermic standard heat of combustion?
1. Combustion of ethane
2. Combustion of propane
3. Combustion of n-butane
4. Combustion of n-pentane
5. Methanol reacts with acetic acid to form methyl acetate and water.
|
Type of Bond |
Bond Disassociation Energy |
|
C – C |
348 |
|
C – H |
415 |
|
C – O |
805 |
|
O = H |
463 |
|
C – O |
360 |
6. Based on the values in the table above, what is the heat of formation of methyl acetate in ![]()
1. 0
2. 464
3. 824
4. 1288
6. At standard temperature and pressure, a chemical process is at equilibrium. What is the free energy of reaction (ΔG) for this process?
1. ΔG > 0
2. ΔG < 0
3. ΔG = 0
4. There is not enough information to determine the free energy of the reaction.
7. For a certain chemical process, ![]() What is the equilibrium constant Keq for this reaction? (Note:
What is the equilibrium constant Keq for this reaction? (Note: ![]() )
)
1. Keq = 1.0
2. Keq = 7.4
3. Keq = 8.9
4. Keq = 10
8. Consider the chemical reaction in the vessel depicted in the following diagram.
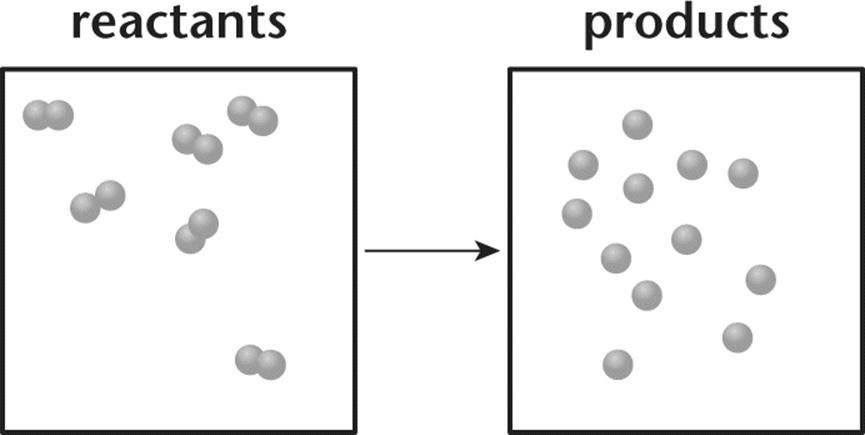
1. The reaction is spontaneous.
2. The reaction is nonspontaneous.
3. The reaction is at equilibrium.
4. There is not enough information to determine if the reaction is spontaneous.
9. Suppose ![]() for a chemical reaction. At 300 K, what is the change in Gibbs free energy in
for a chemical reaction. At 300 K, what is the change in Gibbs free energy in ![]()
1. ΔG = –2000 + (300 K)(8.314)(ln Q)
2. ΔG = –2000 – (300 K)(8.314)(ln Q)
3. ΔG = –2000 + (300 K)(8.314)(log Q)
4. ΔG = –2000 – (300 K)(8.314)(log Q)
10.A chemical reaction has a negative enthalpy and a negative entropy. Which of the following terms necessarily describes this reaction?
1. Exothermic
2. Endothermic
3. Exergonic
4. Endergonic
11.Which of the following statements is true of process that is spontaneous in the forward direction?
1. ΔG > 0 and Keq > Q
2. ΔG > 0 and Keq < Q
3. ΔG < 0 and Keq > Q
4. ΔG < 0 and Keq < Q
12.Which of the following devices would be the most appropriate to use to measure the heat capacity of a liquid?
1. Thermometer
2. Calorimeter
3. Barometer
4. Volumetric flask
13.A reaction coordinate for a chemical reaction is displayed in the graph below.
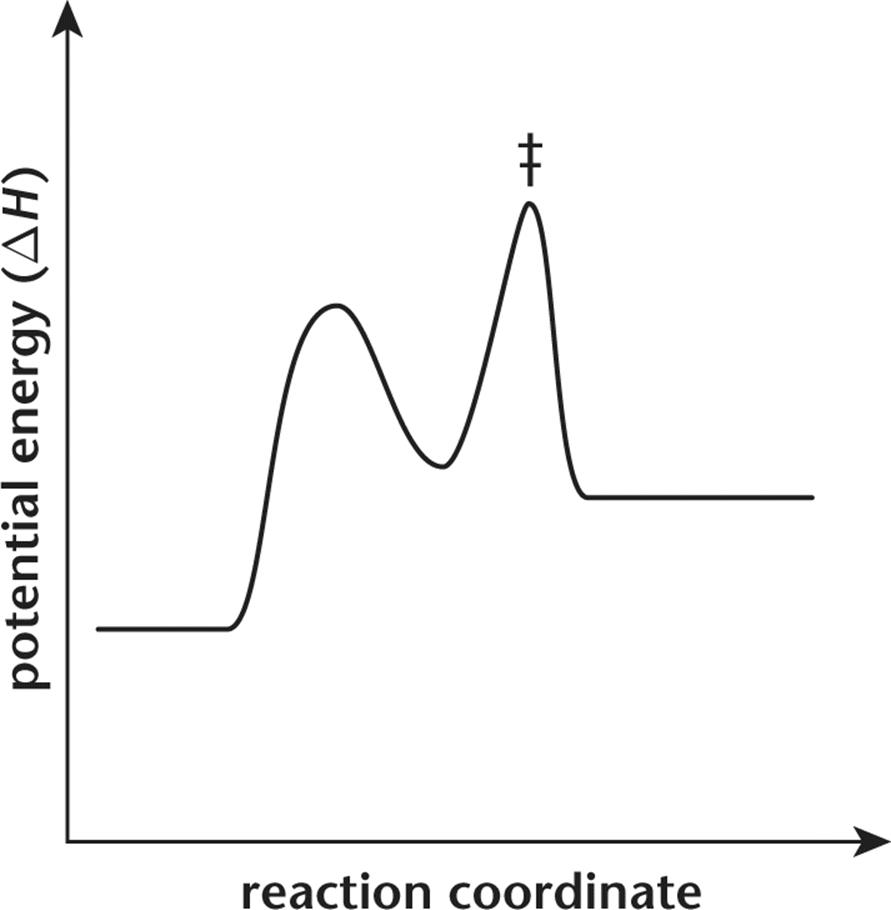
Which of the following terms describes the energy of this reaction?
1. Endothermic
2. Exothermic
3. Endergonic
4. Exergonic
14.Which of the following phase changes is associated with the largest decrease in entropy?
1. Fusion
2. Solidification
3. Deposition
4. Sublimation
15.Explosions are necessarily characterized by:
1. ΔG < 0.
2. ΔH > 0.
3. ΔS < 0.
4. T < 0.
PRACTICE QUESTIONS