MCAT General Chemistry Review
Chapter 8: The Gas Phase
Conclusion
In this chapter, we reviewed the basic characteristics and behaviors of gases. The ideal gas law shows the mathematical relationship among four variables associated with gases: pressure, volume, temperature, and number of moles. We examined special cases of the ideal gas law in which temperature (Boyle’s law), pressure (Charles’s law), or volume (Gay-Lussac’s law) is held constant. Henry’s law helped explain the principles behind dissolution of gases in liquids and gas exchange in biological systems. We also examined Dalton’s law, which relates the partial pressure of a gas to its mole fraction and the sum of the partial pressures of all the gases in a system to the total pressure of the system. The kinetic molecular theory of gases provided the explanation for the behaviors of ideal gases as described by the ideal gas law. Finally, we examined the ways in which real gases deviate from the predicted behaviors of ideal gases. The van der Waals equation of state is a useful equation for correcting deviations caused by molecular interactions and volumes.
From helium-filled balloons to the bubbles of carbon dioxide in a glass of soda, from the pressurized gases used for scuba diving to the air we breathe on land, gases are all around us. And yet, all the different gases that bubble, flow, and settle in and through our daily living experiences behave in remarkably similar ways. Human life is dependent on the exchange of two gases: oxygen and carbon dioxide—to that end, expect that the MCAT will frequently test gases because of their importance in our everyday lives.
Concept Summary
The Gas Phase
· Gases are the least dense phase of matter.
· Gases are fluids and therefore conform to the shapes of their containers.
· Gases are easily compressible.
· Gas systems are described by the variables temperature (T), pressure (P), volume (V), and number of moles (n).
· Important pressure equivalencies include 1 atm = 760 mmHg ≡ 760 torr = 101.325 kPa.
· A simple mercury barometer measures incident (usually atmospheric) pressure. As pressure increases, more mercury is forced into the column, increasing its height. As pressure decreases, mercury flows out of the column under its own weight, decreasing its height.
Ideal Gases
· Standard temperature and pressure (STP) is 273 K (0°C) and 1 atm.
· Equations for ideal gases assume negligible mass and volume of gas molecules.
· Regardless of the identity of the gas, equimolar amounts of two gases will occupy the same volume at the same temperature and pressure. At STP, one mole of an ideal gas occupies 22.4 L.
· The ideal gas law describes the relationship between the four variables of the gas state for an ideal gas.
· Avogadro’s principle is a special case of the ideal gas law for which the pressure and temperature are held constant; it shows a direct relationship between the number of moles of gas and volume.
· Boyle’s law is a special case of the ideal gas law for which temperature and number of moles are held constant; it shows an inverse relationship between pressure and volume.
· Charles’s law is a special case of the ideal gas law for which pressure and number of moles are held constant; it shows a direct relationship between temperature and volume.
· Gay-Lussac’s law is a special case of the ideal gas law for which volume and number of moles are held constant; it shows a direct relationship between temperature and pressure.
· The combined gas law is a combination of Boyle’s, Charles’s, and Gay-Lussac’s laws; it shows an inverse relationship between pressure and volume along with direct relationships between pressure and volume with temperature.
· Dalton’s law of partial pressures states that individual gas components of a mixture of gases will exert individual pressures in proportion to their mole fractions. The total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases.
· Henry’s law states that the amount of gas dissolved in solution is directly proportional to the partial pressure of that gas at the surface of a solution.
Kinetic Molecular Theory
· The kinetic molecular theory attempts to explain the behavior of gas particles. It makes a number of assumptions about the gas particles.
o Gas particles have negligible volume.
o Gas particles do not have intermolecular attractions or repulsions.
o Gas particles undergo random collisions with each other and the walls of the container.
o Collisions between gas particles (and with the walls of the container) are elastic.
o The average kinetic energy of gas particles is directly proportional to temperature.
· Graham’s law describes the behavior of gas diffusion or effusion, stating that gases with lower molar masses will diffuse or effuse faster than gases with higher molar masses at the same temperature.
o Diffusion is the spreading out of particles from high to low concentration.
o Effusion is the movement of gas from one compartment to another through a small opening under pressure.
Real Gases
· Real gases deviate from ideal behavior under high pressure (low volume) and low temperature conditions.
o At moderately high pressures, low volumes, or low temperatures, real gases will occupy less volume than predicted by the ideal gas law because the particles have intermolecular attractions.
o At extremely high pressures, low volumes, or low temperatures, real gases will occupy more volume than predicted by the ideal gas law because the particles occupy physical space.
o The van der Waals equation of state is used to correct the ideal gas law for intermolecular attractions (a) and molecular volume (b).
Answers to Concept Checks
· 8.1
1. Gases are compressible fluids with rapid molecular motion, large intermolecular distances, and weak intermolecular forces.
2. At the top of the mountain, atmospheric pressure is lower, causing the column to fall. Under water, hydrostatic pressure is exerted on the barometer in addition to atmospheric pressure, causing the column to rise.
3. STP: T = 273 K (0°C), P = 1 atm
4. Standard conditions: T = 298 K (25°C), P = 1 atm, concentrations = 1 M
· 8.2
1. 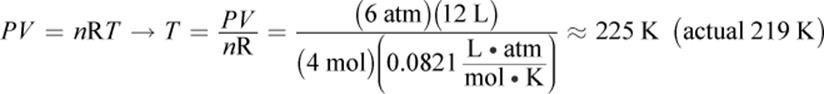
2. 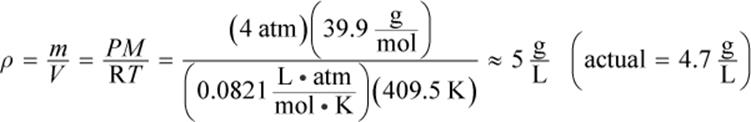
3. 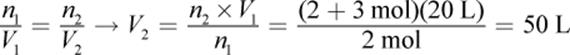
4. 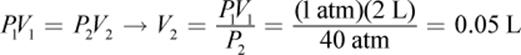
5. 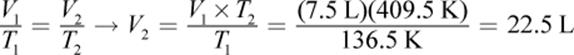
6. 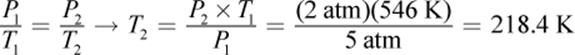
1. There are twelve total moles of gas, so the mole fractions of each gas are:
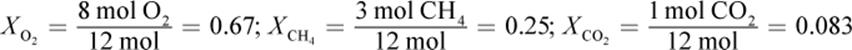
Then multiply each mole fraction by the total pressure to get the partial pressures:
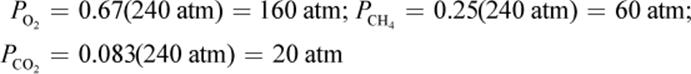
2. High pressures of carbon dioxide gas are forced on top of the liquid in sodas, increasing its concentration in the liquid.
· 8.3
1. Assumptions in the kinetic molecular theory include: negligible volume of gas particles, no intermolecular forces, random motion, elastic collisions, and proportionality between absolute temperature and energy
2. 
3. 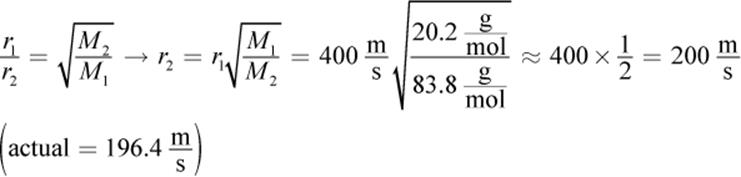
4. The rotten egg odor (hydrogen sulfide) first, almond (benzaldehyde) next, and wintergreen (methyl salicylate) last. Because all of the gases have the same temperature, they have the same kinetic energy; thus, the lightest molecules travel the fastest.
· 8.4
1. Real gas molecules have nonnegligible volume and attractive forces. Real gases deviate from ideal gases at high pressure (low volume) and low temperature.
2. According to the van der Waals equation, if a is increased while b remains negligible, the correction term 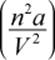 gets larger, and the pressure or volume must drop to compensate.
gets larger, and the pressure or volume must drop to compensate.
3. Increasing the volume of gas molecules while keeping attraction negligible makes the term V – nb smaller; thus, the pressure or volume must rise to compensate.
Equations to Remember
(8.1) Ideal gas law: PV = nRT
(8.2) Density of a gas: ![]()
(8.3) Combined gas law: 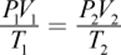
(8.4) Avogadro’s principle: 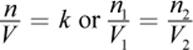
(8.5) Boyle’s law: PV = k or P1V1 = P2V2
(8.6) Charles’s law: 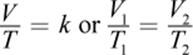
(8.7) Gay-Lussac’s law: 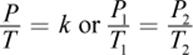
(8.8) Dalton’s law (total pressure from partial pressures): PT = PA + PB + PC + …
(8.9) Dalton’s law (partial pressure from total pressure): PA = XA PT
(8.10) Henry’s law: [A] = kH × PA or 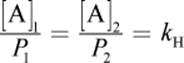
(8.11) Average kinetic energy of a gas: ![]()
(8.12) Root-mean-square speed: 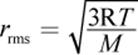
(8.13) Graham’s law: 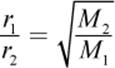
(8.14) Van der Waals equation of state:
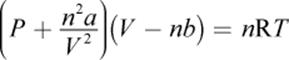
Shared Concepts
· Biology Chapter 6
o The Respiratory System
· General Chemistry Chapter 3
o Bonding and Chemical Interactions
· General Chemistry Chapter 6
o Equilibrium
· Physics and Math Chapter 2
o Work and Energy
· Physics and Math Chapter 3
o Thermodynamics
· Physics and Math Chapter 4
o Fluids