MCAT General Chemistry Review
Chapter 9: Solutions
9.1 Nature of Solutions
Many important chemical reactions, both in the laboratory and in nature, take place in solutions, including almost all reactions in living organisms. Solutions are homo-geneous (the same throughout) mixtures of two or more substances that combine to form a single phase, usually the liquid phase. The MCAT will focus almost exclusively on solids dissolved into aqueous solutions, but it’s important to remember that solutions can be formed from different combinations of the three phases of matter. For example, gases can be dissolved in liquids (carbonating soda); liquids can be dissolved in other liquids (ethanol in water); solids can even be dissolved in other solids (metal alloys). Incidentally, gases “dissolved” into other gases can be thought of as solutions, but are more properly defined only as mixtures because gas molecules do not interact all that much chemically, as described by the kinetic molecular theory of gases. As a point of clarification: all solutions are considered mixtures, but not all mixtures are considered solutions.
A solution consists of a solute (such as NaCl, NH3, C6H12O6, or CO2) dissolved (dispersed) in a solvent (such as H2O, benzene, or ethanol). The solvent is the component of the solution that remains in the same phase after mixing. If the two substances are already in the same phase (for example, a solution of two liquids), the solvent is the component present in greater quantity. If the two same-phase components are in equal proportions in the solution, then the component that is more commonly used as a solvent in other contexts is considered the solvent. Solute molecules move about freely in the solvent and interact with it by way of intermolecular forces such as ion–dipole, dipole–dipole, or hydrogen bonding. Dissolved solute molecules are also relatively free to interact with other dissolved molecules of different chemical identities; consequently, chemical reactions occur easily in solution.
SOLVATION
Solvation is the electrostatic interaction between solute and solvent molecules. This is also known as dissolution, and when water is the solvent, it can be called hydration. Solvation involves breaking intermolecular interactions between solute molecules and between solvent molecules and forming new intermolecular interactions between solute and solvent molecules together, as shown in Figure 9.1 (which was also shown in Chapter 4 of MCAT General Chemistry Review in the context of ions).
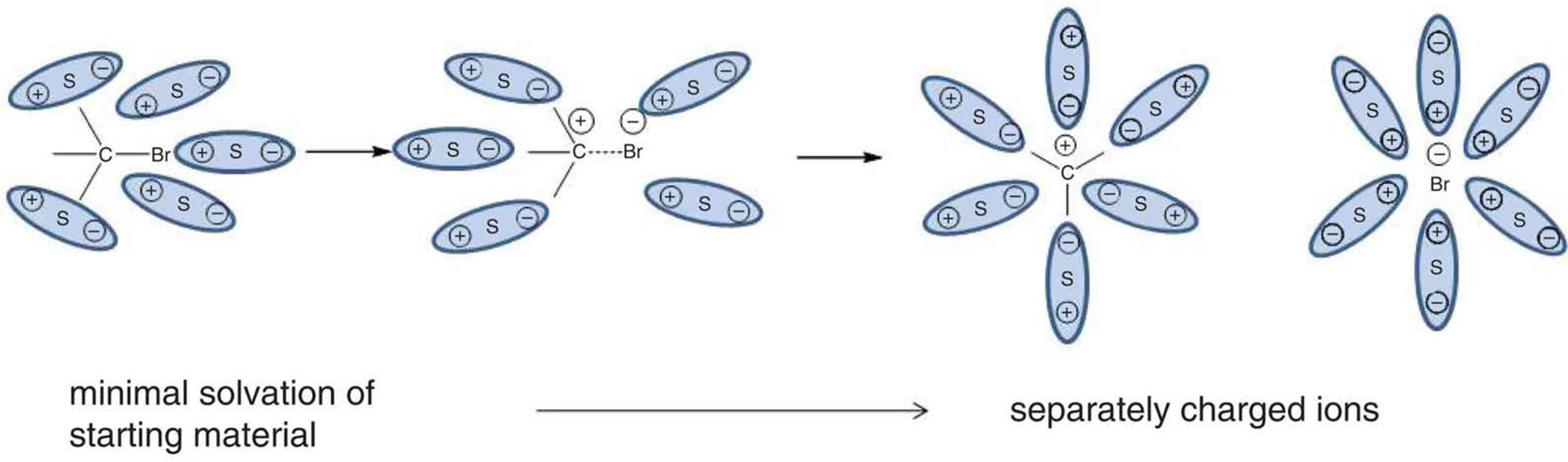 Figure 9.1. Solvation of a Polar Covalent Compound S indicates a solvent particle
Figure 9.1. Solvation of a Polar Covalent Compound S indicates a solvent particle
When the new interactions are stronger than the original ones, solvation is exothermic, and the process is favored at low temperatures. The dissolution of gases into liquids, such as CO2 into water, is an exothermic process because the only significant interactions that must be broken are those between water molecules—CO2, as a gas, demonstrates minimal intermolecular interaction. Le Châtelier’s principle tells us this is the reason that lowering the temperature of a liquid favors solubility of a gas in the liquid.
When the new interactions are weaker than the original ones, solvation is endothermic and the process is favored at high temperatures. Most dissolutions are of this type. Two such examples have already been given: dissolving ammonium nitrate or sugar into water. Because the new interactions between the solute and solvent are weaker than the original interactions between the solute molecules and between the solvent molecules, energy (heat) must be supplied to facilitate the formation of these weaker, less stable interactions. Sometimes the overall strength of the new interactions is approximately equal to the overall strength of the original interactions. In this case, the overall enthalpy change for the dissolution is close to zero. These types of solutions approximate the formation of an ideal solution, for which the enthalpy of dissolution is equal to zero.
The spontaneity of dissolution is dependent not only on the enthalpy change; solutions may form spontaneously for both endothermic and exothermic dissolutions. The second property that contributes to the spontaneity of dissolution is the entropy change that occurs in the process. At constant temperature and pressure, entropy always increases upon dissolution. As with any process, the spontaneity of dissolution depends on the change in Gibbs free energy: spontaneous processes are associated with a decrease in free energy, while nonspontaneous processes are associated with an increase in free energy. Thus, whether or not dissolution will happen spontaneously depends on both the change in enthalpy and the change in entropy for the solute and solvent of the system.
BRIDGE
Proteins dissolve in solution with their most hydrophilic amino acids on the outside and hydrophobic amino acids on the inside because this maximizes the increase in entropy during dissolution. As described in Chapter 1 of MCAT Biochemistry Review, a protein dissolves by forming a solvation layer.
Consider, for example, the formation of another common solution: sodium chloride dissolved in water. When NaCl dissolves in water, its component ions dissociate from each other and become surrounded by water molecules. For this new interaction to occur, ion–ion interactions between Na+ and Cl− must be broken, and hydrogen bonds between water molecules must also be broken. This step requires energy and is therefore endothermic. Because water is polar, it can interact with each of the component ions through ion–dipole interactions: the partially positive hydrogen end of the water molecules will surround the Cl− ions, and the partially negative oxygen end of the water molecules will surround the Na+ ions, as shown in Figure 9.2. The formation of these ion–dipole bonds is exothermic, but the magnitude is slightly less than the energy required to break the ionic bonds and hydrogen bonds. As a result, the overall dissolution of table salt into water is endothermic 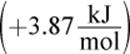 and favored at high temperatures.
and favored at high temperatures.
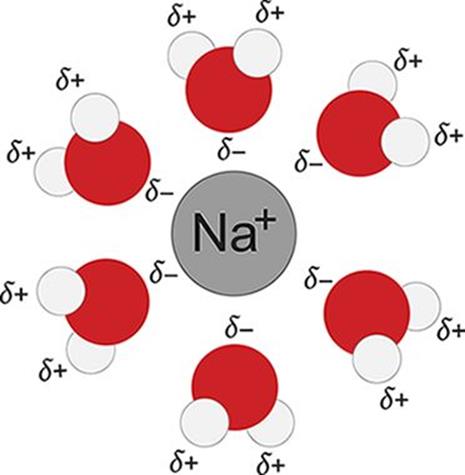 Figure 9.2. Solvation of Na+ Ions in Aqueous Solutions
Figure 9.2. Solvation of Na+ Ions in Aqueous Solutions
We’ve considered the enthalpy change for the formation of a sodium chloride solution, and now we need to examine the entropy change. Remember that entropy can be thought of as the degree to which energy is dispersed throughout a system or the amount of energy distributed from the system to the surroundings at a given temperature. Another way to understand entropy is the measure of molecular disorder, or the number of energy microstates available to a system at a given temperature. When solid sodium chloride dissolves into water, the rigidly ordered arrangement of the sodium and chloride ions is broken up as the ion–ion interactions are disrupted and new ion–dipole interactions with the water molecules are formed. The ions, freed from their lattice arrangement, have a greater number of energy microstates available to them (in simpler terms, they are freer to move around in different ways), and consequently, their energy is more distributed and their entropy increases. The water, however, becomes more restricted in its movement because it is now interacting with the ions. The number of energy microstates available to it (that is, the water molecules’ ability to move around in different ways) is reduced, so the entropy of the water decreases. In the end, the increase in the entropy experienced by the dissolved sodium chloride is greater than the decrease in the entropy experienced by the water, so the overall entropy change is positive—energy is, overall, dispersed by the dissolution of sodium chloride in water. Because of the relatively low endothermicity and relatively large positive change in entropy, sodium chloride will spontaneously dissolve in liquid water (ΔG = ΔH – TΔS).
SOLUBILITY
We often want to know more than just whether or not dissolution of a solute into a solvent will be spontaneous or nonspontaneous—we also want to know how much solute will dissolve into a given solvent. The solubility of a substance is the maximum amount of that substance that can be dissolved in a particular solvent at a given temperature. When this maximum amount of solute has been added, the dissolved solute is in equilibrium with its undissolved state, and we say that the solution is saturated. If more solute is added, it will not dissolve. For example, at 25°C, a maximum of 90.9 g glucose will dissolve in 100 mL H2O. Thus, the solubility of glucose is ![]() If more glucose is added to an already saturated glucose solution, it will not dissolve but rather will remain in solid form, precipitating to the bottom of the container. A solution in which the proportion of solute to solvent is small is said to be dilute, and one in which the proportion is large is said to be concentrated. Note that both dilute and concentrated solutions are still considered unsaturated if the maximum equilibrium concentration (saturation) has not yet been reached.
If more glucose is added to an already saturated glucose solution, it will not dissolve but rather will remain in solid form, precipitating to the bottom of the container. A solution in which the proportion of solute to solvent is small is said to be dilute, and one in which the proportion is large is said to be concentrated. Note that both dilute and concentrated solutions are still considered unsaturated if the maximum equilibrium concentration (saturation) has not yet been reached.
The solubility of substances in different solvents is ultimately a function of thermodynamics. When the change in Gibbs free energy for the dissolution reaction is negative at a given temperature, the process will be spontaneous, and the solute is said to be soluble. When the change in Gibbs free energy is positive, the process will be nonspontaneous, and the solute is said to be insoluble. Some solute–solvent systems have negative changes in free energy with very large magnitudes, so the equilibrium reaction strongly favors the dissolution of the solute. In general, solutes are considered soluble if they have a molar solubility above 0.1 M in solution. Others have only slightly negative changes in free energy, so the equilibrium position lies closer to the undissociated (reactants) side of the reaction. Those solutes that dissolve minimally in the solvent (molar solubility under 0.1 M) are called sparingly soluble salts.
AQUEOUS SOLUTIONS
The most common type of solution is the aqueous solution, in which the solvent is water. The aqueous state is denoted by the symbol (aq). Aqueous solutions rely on the interactions between water molecules and solutes in solutions. We have mentioned previously that hydration is often the process through which dissolution occurs. It is also important to note that in some solutions, such as acids, the formation of a complex called the hydronium ion (H3O+) can occur. This is facilitated by the transfer of a hydrogen ion (H+) from a molecule in solution to a water molecule (H2O). The reaction of acetic acid (H+ donor) with water is shown in Figure 9.3.
CH3COOH (aq) + H2O (l) ⇋ CH3COO− (aq) + H3O+ (aq)
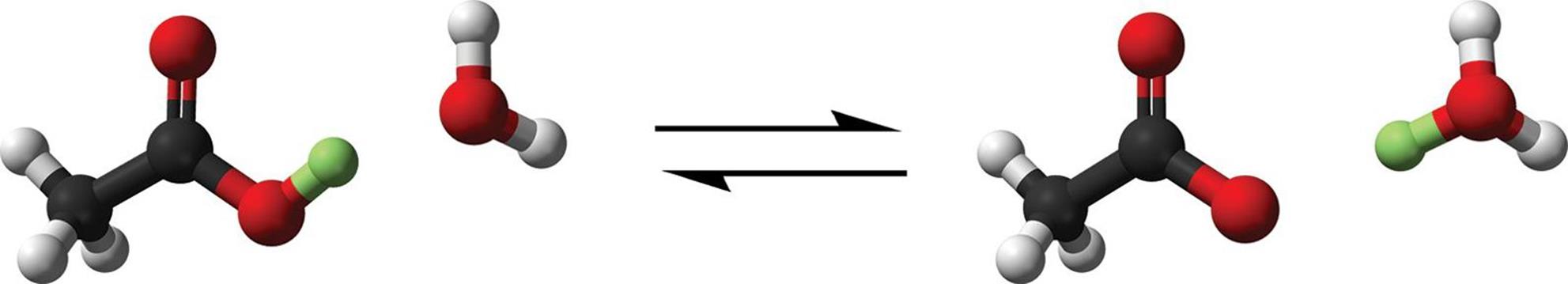 Figure 9.3. Transfer of a Proton in Solution, Forming the Hydronium Ion The transferred proton is highlighted in green.
Figure 9.3. Transfer of a Proton in Solution, Forming the Hydronium Ion The transferred proton is highlighted in green.
It is important to realize that H+ is never found alone in solution because a free proton is difficult to isolate; rather, it is found bound to an electron pair donor (carrier) molecule such as a water molecule. This is an example of a coordinate covalent bond. The hydronium ion and its effects on the solubilities of other compounds using Le Châtelier’s principle will be described further in Chapter 10 of MCAT General Chemistry Review.
Because aqueous solutions are so common and so important to biological systems, the MCAT focuses on them above all others. In aqueous solutions, there are seven general solubility rules:
1. All salts containing ammonium (NH4+) and alkali metal (Group 1) cations are water-soluble.
2. All salts containing nitrate (NO3−) and acetate (CH3COO−) anions are water-soluble.
3. Halides (Cl−, Br−, I−), excluding fluorides, are water-soluble, with the exceptions of those formed with Ag+, Pb2+, and HG22+.
4. All salts of the sulfate ion (SO42−) are water-soluble, with the exceptions of those formed with Ca2+, Sr2+, Ba2+, and Pb2+.
5. All metal oxides are insoluble, with the exception of those formed with the alkali metals, ammonium, and CaO, SrO, and BaO, all of which hydrolyze to form solutions of the corresponding metal hydroxides.
6. All hydroxides are insoluble, with the exception of those formed with the alkali metals, ammonium, and Ca2+, Sr2+, and Ba2+.
7. All carbonates (CO32−), phosphates (PO43−), sulfides (S2–), and sulfites (SO32−) are insoluble, with the exception of those formed with the alkali metals and ammonium.
MCAT EXPERTISE
Because most solutions in the real world involve water as the solvent, it is not a surprise that they are common on the MCAT. These solubility rules are not bad to know, but memorizing them all may be a little excessive. It is never a bad thing to know facts, but being able to apply them is more important. Know rules 1 and 2 for sure, and be aware of some of the more common insoluble exceptions, like Pb2+ and Ag+.
The MCAT will not expect memorization of all of the solubility rules, but it is worth knowing two absolutes: all salts of Group 1 metals, and all nitrate salts are soluble. Otherwise, familiarity with rules listed above will suffice—the MCAT generally supplies solubility information for most compounds. Sodium and nitrate ions are generally used as counterions to what is actually chemically important; for example, if a pH problem gives a sodium formate concentration of 0.10 M, it is really indicating that the concentration of the formate ion is 0.10 M because the sodium ion concentration does not affect pH. The only time one needs to worry about the nitrate ion concentration is in an oxidation–reduction reaction, for the nitrate ion can function—although only weakly—as an oxidizing agent. In all other cases, only focus on the cation as the chemically reacting species.
COMPLEX ION FORMATION
We have mentioned the hydronium ion as a complex that forms in acidic solutions, but it is worthwhile to mention that there are even more varied forms of complex ions that can appear in solution. By definition, a complex ion—or coordination compound—refers to a molecule in which a cation is bonded to at least one electron pair donor (which could include the water molecule). The electron pair donor molecules are called ligands. An example of such a complexation reaction is shown for the tetraaquadioxouranyl cation, which has water (aqua–) and oxygen (oxo–) ligands, in Figure 9.4.
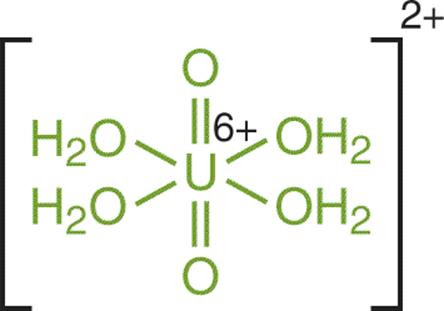 Figure 9.4. Structure of the Tetraaquadioxouranyl Complex Cation Water and oxygen act as ligands with a U6+ cation.
Figure 9.4. Structure of the Tetraaquadioxouranyl Complex Cation Water and oxygen act as ligands with a U6+ cation.
Complexes are held together with coordinate covalent bonds, in which an electron pair donor (a Lewis base) and an electron pair acceptor (a Lewis acid) form very stable Lewis acid–base adducts. Most general chemistry courses do not stress the biological importance of coordination compounds. However, complex ions have profound biological applications in macromolecules such as proteins. For instance, many active sites of proteins utilize complex ion binding and transition metal complexes to carry out their function. One classic example is the iron cation in hemoglobin, which can carry oxygen, carbon dioxide, and carbon monoxide as ligands, as shown in Figure 9.5.
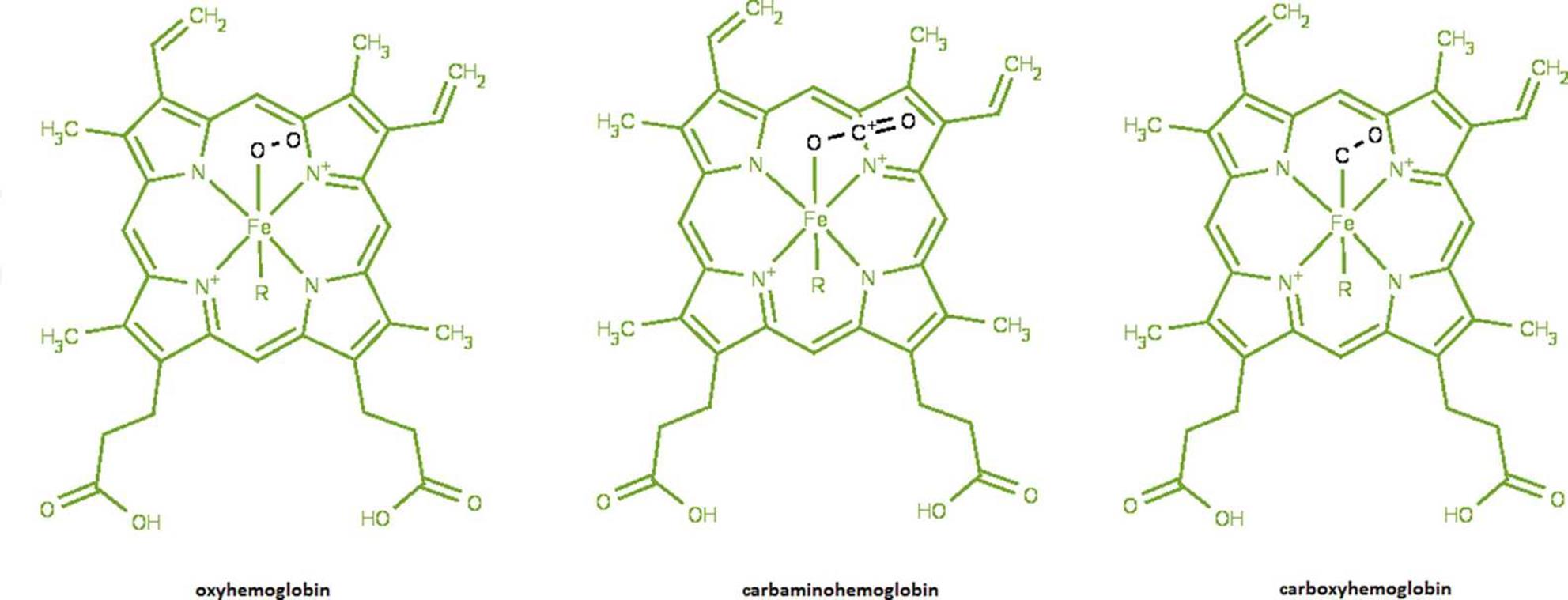 Figure 9.5. Hemoglobin Is a Classic Example of Biochemical Complex Formation The iron in hemoglobin can bind various gases, leading to the formation of oxyhemoglobin (O2), carbaminohemoglobin (CO2), and carboxyhemoglobin (CO).
Figure 9.5. Hemoglobin Is a Classic Example of Biochemical Complex Formation The iron in hemoglobin can bind various gases, leading to the formation of oxyhemoglobin (O2), carbaminohemoglobin (CO2), and carboxyhemoglobin (CO).
Many coenzymes (vitamins) and cofactors also contain complexes of transition metals, such as cobalamin (vitamin B12), shown in Figure 9.6. The presence of a transition metal allows coenzymes and cofactors to bind other ligands or assist with electron transfer.
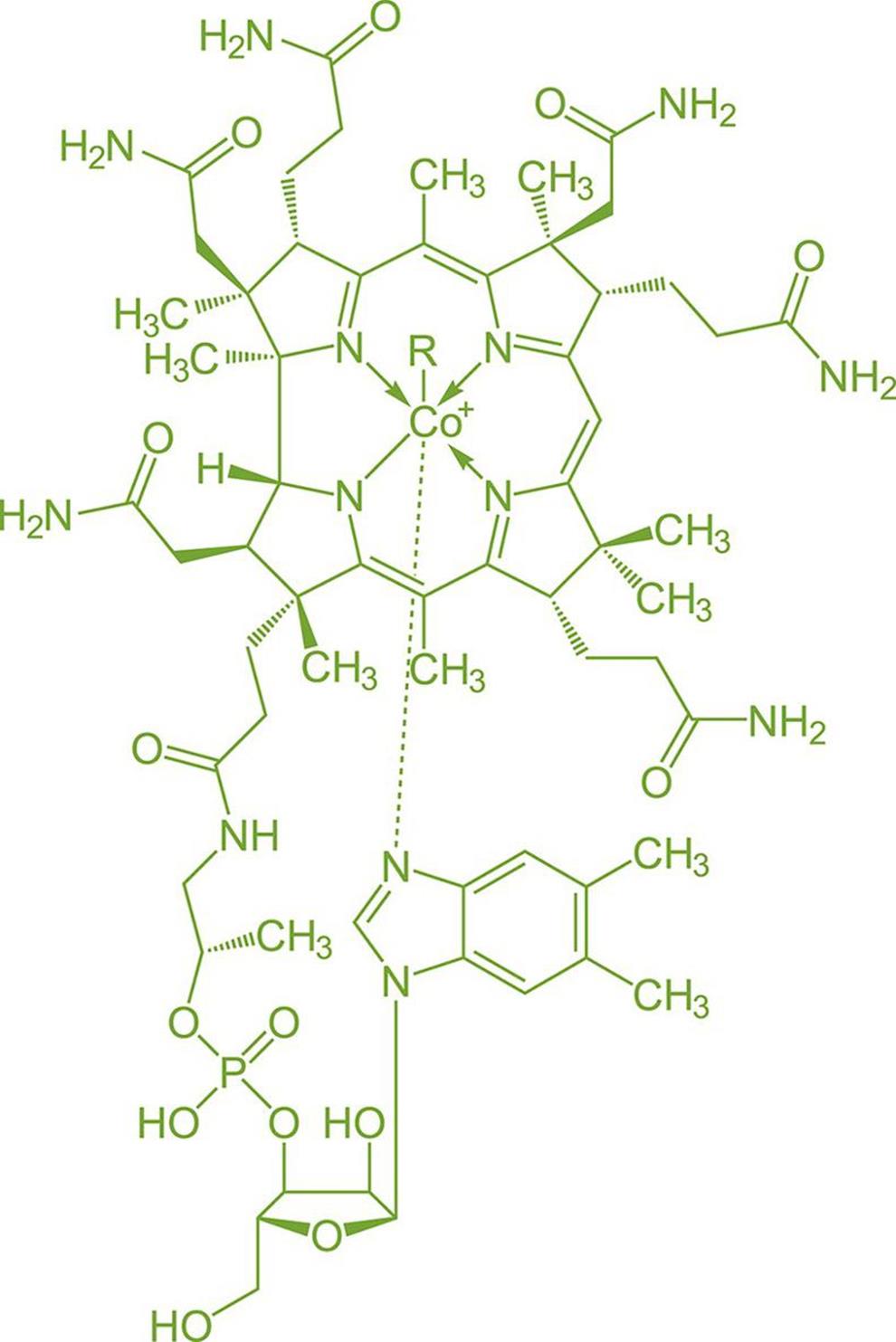 Figure 9.6. Cobalamin (Vitamin B12) Contains a Cobalt Complex
Figure 9.6. Cobalamin (Vitamin B12) Contains a Cobalt Complex
Physical and chemical properties of complex ions are diverse, including a wide range of solubilities and varied chemical reactions. Inorganic complex ions are often fun to characterize because they tend to have vibrant, distinctive colors, as shown in Figure 9.7.
 Figure 9.7. Nickel(II) Ion Complexes with Distinctive Colors From left to right: hexaamminenickel(II), tris(ethylenediamine)nickel(II), tetrachloronickelate(II), and hexaaquanickel(II)
Figure 9.7. Nickel(II) Ion Complexes with Distinctive Colors From left to right: hexaamminenickel(II), tris(ethylenediamine)nickel(II), tetrachloronickelate(II), and hexaaquanickel(II)
In some complexes, the central cation can be bonded to the same ligand in multiple places. This is called chelation, and it generally requires large organic ligands that can double back to form a second (or even third) bond with the central cation. Chelation therapy is often used to sequester toxic metals (lead, arsenic, mercury, and so on). Even biologically necessary metals, such as iron, can be toxic in overload states; an example of iron being chelated is shown in Figure 9.8.
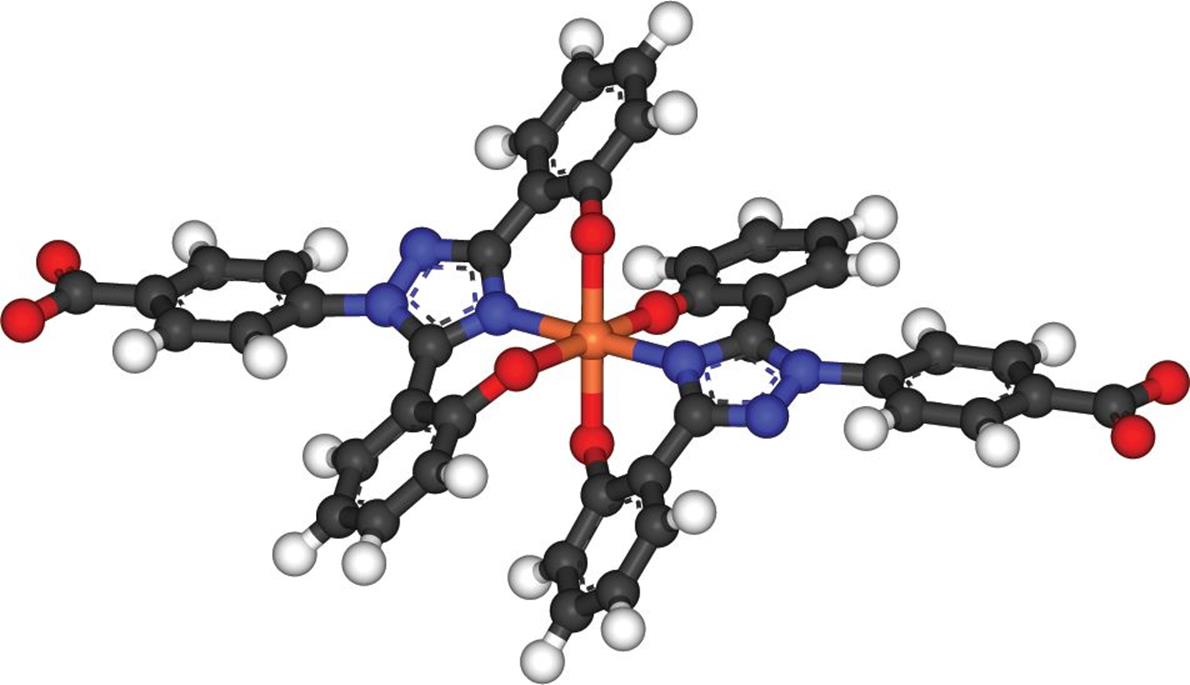 Figure 9.8. Chelation of Iron with Two Molecules of Deferasirox
Figure 9.8. Chelation of Iron with Two Molecules of Deferasirox
MCAT Concept Check 9.1:
Before you move on, assess your understanding of the material with these questions.
1. Describe the process of solvation.
2. Describe the differences between solubility and saturation:
·
·
3. What is one way in which solubility of a compound can be increased?
4. Name two ions that form salts that are always soluble:
·
·