MCAT General Chemistry Review
Chapter 9: Solutions
Answers and Explanations
1. A
The equation ΔTb = iKbm can be used to solve this problem. The change in boiling point is 101.11 − 100 = 1.11°C. Then, we can plug that into:
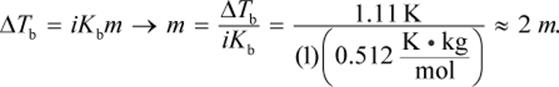
The van ’t Hoff factor for this solute is 1 because the molecule does not dissociate into smaller components. Then, we can convert to grams of solute using the definition of molality:

The mass used in this equation is 0.1 kg because 100 mL of water has a mass of 0.1 kg. Then, we can determine the molar mass:
![]()
which is closest to choice (A).
2. DAll three choices can make a solution as long as the two components create a mixture that is of uniform appearance (homogenous). Hydrogen in platinum is an example of a gas in a solid. Brass and steel are examples of homogenous mixtures of solids. The air we breathe is an example of a homogenous mixture of gases; while these are more commonly simply referred to as mixtures, they still fit the criteria of a solution.
3. BBenzene and toluene are both organic liquids and have very similar properties. They are both nonpolar and are almost exactly the same size. Raoult’s law states that ideal solution behavior is observed when solute–solute, solvent–solvent, and solute–solvent interactions are all very similar. Therefore, benzene and toluene in solution will be predicted to behave as a nearly ideal solution.
4. CMelting point depresses upon solute addition, making choices (A) and (B) incorrect. Solute particles interfere with lattice formation, the highly organized state in which solid molecules align themselves. Colder-than-normal conditions are necessary to create the solid structure.
5. DThe first step will most likely be endothermic because energy is required to break molecules apart. The second step is also endothermic because the intermolecular forces in the solvent must be overcome to allow incorporation of solute particles. The third step will most likely be exothermic because polar water molecules will interact with the dissolved ions, creating a stable solution and releasing energy.
6. CCaS will cause the most negative ΔS°soln because the Ca2+ and S2− ions have the highest charge density compared to the other ions. All of the other ions have charges of +1 or –1,whereas Ca2+ and S2− each have charges with a magnitude of 2.
7. BFormation of complex ions between silver ions and ammonia will cause more molecules of solid AgCl to dissociate. The equilibrium is driven toward dissociation because the Ag+ ions are essentially being removed from solution when they complex with ammonia. This rationale is based upon Le Châtelier’s principle, stating that when a chemical equilibrium experiences a change in concentration, the system will shift to counteract that change.
8. BThe mass percent of a solute equals the mass of the solute divided by the mass of the total solution times 100%. To find the mass of the solution, first find the mass of the solvent, water. Multiplying the volume of the water by the density gives a mass of 292.5 grams of water. Adding 100 grams of sugar yields a solution with a mass of 392.5 grams. Next, divide 100 grams of sugar by 392.5 grams and multiply by 100 to get 25.5%. Choice (A) results if water’s density at 80°C is assumed to be ![]() If we had forgotten to add the solute’s mass to the solvent’s, we would have calculated 34.2%, which is choice (D). Choice (C) neglects both the addition step and the correct density value.
If we had forgotten to add the solute’s mass to the solvent’s, we would have calculated 34.2%, which is choice (D). Choice (C) neglects both the addition step and the correct density value.
9. AMixtures that have a higher vapor pressure than predicted by Raoult’s law have stronger solvent–solvent and solute–solute interactions than solvent–solute interactions. Therefore, particles do not want to stay in solution and more readily evaporate, creating a higher vapor pressure than an ideal solution. Two liquids that have different properties, like hexane (hydrophobic) and ethanol (hydrophilic, small) in Choice (A), would not have many interactions with each other and would cause positive deviation. Choices (B) and (C) are composed of liquids that are similar to one another and would not show significant deviation from Raoult’s law. Choice (D) contains two liquids that would interact very well with each other, which would actually cause a negative deviation from Raoult’s law—when attracted to one other, liquids prefer to stay in liquid form and have a lower vapor pressure than predicted by Raoult’s law.
10.ADissolution is governed by enthalpy and entropy, which are related by the equation ΔG°soln = ΔH°soln − TΔS°soln. The cooling of the solution indicates that heat is used up in this bond-breaking reaction. In other words, dissolution is endothermic, and ΔH is positive. The reaction is occurring spontaneously, so ΔG must be negative. The only way that a positive ΔH can result in a negative ΔG is if entropy, ΔS, is a large, positive value as in choice (A). Conceptually, that means that the only way the solid can dissolve is if the increase in entropy is great enough to overcome the increase in enthalpy. Choice (B) is incorrect because it is clearly stated in the question stem that KCl dissolves; further, all salts of Group 1 metals are soluble. Choice (C) is incorrect because ΔS°soln must be positive in order for KCl to dissolve. Finally, choice (D) is incorrect because solute dissolution would cause the boiling point to elevate, not depress. It is also not a piece of evidence that could be found simply by observing the beaker’s temperature change.
11.B
The equation to determine the change in boiling point of a solution is as follows: ΔTb = iKbm.m is the molality of the solution, and Kb is the boiling point elevation constant. In this case, the solvent is always water, so Kb will be the same for each solution. What we do need to know is how many particles dissociate from each of the original species. This is referred to as the van ’t Hoff factor (i) and is multiplied by our molality to demonstrate a normality (the concentration of the species of interest—in this case, all particles). We’ll use normality values to determine which will cause the greatest change in boiling point.
|
Species |
Number of Moles |
Number of Dissolved Particles |
i × m (Normality) |
|
CaSO4 |
0.46 |
2 |
0.92 |
|
Fe(NO3)3 |
0.54 |
4 |
2.16 |
|
CH3COOH |
1.09 |
Between 1 and 2 (acetic acid is a weak acid and a low percentage of the molecules will dissociate into 2 particles) |
Between 1.09 and 2.18 |
|
C12H22O11 |
1.11 |
1 |
1.11 |
It looks like we need to decide between iron(III) nitrate and acetic acid. No more calculations are necessary because the fact that acetic acid is a weak acid tells us that only a few particles will dissociate into H+ and acetate. Therefore, the normality of the acetic acid will be much closer to 1.09 than 2.18.
12.AThe solubility of gases in liquids is directly proportional to the atmospheric pressure. This is called Henry’s law, and is discussed in Chapter 8 of MCAT General Chemistry Review. Even without the formula, we know there is a direct proportionality between partial pressure and solubility. Because 0.800 atm is 80% of the pressure at sea level (1 atm), oxygen’s solubility will be 80% of 1.25 × 10−3, which is 1.00 × 10−3 M.
13.C
30 ppb of Pb2+ is equivalent to 30 grams of Pb2+ in 109 grams of solution; given the extremely low concentration of lead, we can assume the mass of the water is around 109 grams, as well. From here, this is simply a dimensional analysis question. The units we want at the end are moles per liter (molarity), so we must covert from grams of lead to moles of lead and grams of water to liters of water:
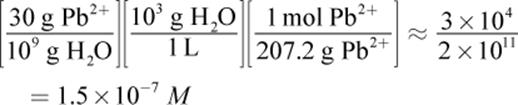
14.CThallium(III) hydroxide has a lower Ksp value than cobalt(III) hydroxide. It is important to note that one can assume the molar solubility of thallium(III) hydroxide is lower than cobalt(III) hydroxide only because both salts have a formula MX3 (one of one particle, three of another). When the solutions are mixed, [OH−] is at saturation levels in the cobalt solution—which is higher than saturation levels in the thallium solution. Therefore, the ion product for thallium(III) hydroxide is higher than its solubility product constant, and the system will shift left to form solid thallium(III) hydroxide, which precipitates.
15.D
The solubility of AgBr can be determined using the Ksp value given in the equation. Some amount of AgBr will dissolve. If we call this amount x, then there will be x amount of silver(I) formed and x amount of bromide—which is added to the 0.0010 M already present from NaBr.
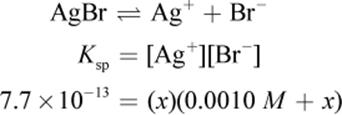
Remember that x is always very small. Even though 0.0010 M is also very small, it will still be much larger than the value of x on Test Day. Thus, the math can be simplified to: 7.7 × 10−13 = (x)(0.001). Therefore, x, the molar solubility, is 7.7 × 10−10, which looks like choice (C).However, the units are grams per liter, not molarity. Thus, we must multiply by the molar mass 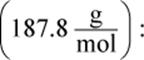
![]()
which is close to choice (D).