MCAT General Chemistry Review
Chapter 10: Acids and Bases
10.3 Polyvalence and Normality
The relative acidity or basicity of an aqueous solution is determined by the relative concentrations of acid and base equivalents. An acid equivalent is equal to one mole of H+ (or, more properly, H3O+) ions; a base equivalent is equal to one mole of OH− ions. Some acids and bases arepolyvalent; that is, each mole of the acid or base liberates more than one acid or base equivalent. Under the Brønsted–Lowry definition, such acids or bases could also be termed polyprotic. For example, the divalent diprotic acid H2SO4 undergoes the following dissociation in water:
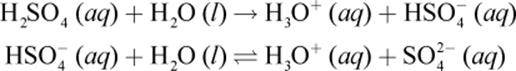
One mole of H2SO4 produces two acid equivalents (2 moles of H3O+). Notice that the first dissociation goes to completion, but the second dissociation reaches an equilibrium state. The acidity or basicity of a solution depends on the concentration of acidic or basic equivalents that can be liberated. The quantity of acidic or basic capacity is directly indicated by the solution’s normality, described in Chapter 9 of MCAT General Chemistry Review. For example, each mole of H3PO4 yields three moles (equivalents) of H3O+. Therefore, a 2 M H3PO4 solution would be 6 N.
Another measurement useful for acid–base chemistry is gram equivalent weight. Chapter 4 of MCAT General Chemistry Review defined and discussed this term extensively. The gram equivalent weight is the mass of a compound that produces one equivalent (one mole of charge). For example, H2SO4 (molar mass: ![]() ) is a divalent acid, so each mole of the acid compound yields two acid equivalents. The gram equivalent weight is 98 ÷ 2 = 49 grams. That is, the complete dissociation of 49 grams of H2SO4 will yield one acid equivalent (one mole of H3O+). Common polyvalent acids include H2SO4, H3PO4, and H2CO3. Common polyvalent bases include Al(OH)3, Ca(OH)2, and Mg(OH)2.
) is a divalent acid, so each mole of the acid compound yields two acid equivalents. The gram equivalent weight is 98 ÷ 2 = 49 grams. That is, the complete dissociation of 49 grams of H2SO4 will yield one acid equivalent (one mole of H3O+). Common polyvalent acids include H2SO4, H3PO4, and H2CO3. Common polyvalent bases include Al(OH)3, Ca(OH)2, and Mg(OH)2.
BRIDGE
To review normality in more detail, revisit the calculations performed in Chapter 4 of MCAT General Chemistry Review. These are critical calculations for polyvalent acids and bases.
MCAT Concept Check 10.3:
Before you move on, assess your understanding of the material with these questions.
1. What species are considered the equivalents for acids and bases, respectively?
· Acids:
· Bases:
2. Calculate the normality of the following solutions:
· 2 M Al(OH)3:
· 16 M H2SO4: