MCAT General Chemistry Review
Chapter 10: Acids and Bases
Conclusion
In this chapter, we have reviewed the important principles of acid–base chemistry. We clarified the differences among the three definitions of acids and bases, including the nomenclature of some common Arrhenius acids. We investigated important properties of acids and bases, including the important acid–base behavior of water (autoionization) and hydrogen ion equilibria. We explained the mathematics of the pH and pOH logarithmic scales and demonstrated a useful Test Day shortcut for approximating the logarithmic value of hydrogen ion or hydroxide ion concentrations. Strong acids and bases are defined as compounds that completely dissociate in aqueous solutions, and weak acids and bases are compounds that only partially dissociate (to an equilibrium state). We discussed neutralization and salt formation upon reaction of acids and bases, and finally, we applied our fundamental understanding of acid–base reactivity to titrations and buffer systems. Titrations are useful for determining the concentration of a known acid or base solution. Weak acid and weak base buffers are useful for minimizing changes in pH upon addition of strong acid or base.
You’ve just accomplished a major task in the overall effort to earn points on Test Day. It’s okay if you didn’t understand everything on this first pass. Go back and review the concepts that were challenging for you and then complete the questions at the end of the chapter and MCAT practice passages to test your knowledge. Don’t be alarmed if you find yourself reviewing parts or all of a chapter a second or third time—repetition is the key to success.
You are now two chapters away from completing this review of general chemistry. While we don’t want to offer our congratulations prematurely, we want to acknowledge all the hard work you’ve invested in this process. Keep it up: success on Test Day is within your reach!
Concept Summary
Definitions
· Arrhenius acids dissociate to produce an excess of hydrogen ions in solution. Arrhenius bases dissociate to produce an excess of hydroxide ions in solution.
· Brønsted–Lowry acids are species that can donate hydrogen ions. Brønsted–Lowry bases are species that can accept hydrogen ions.
· Lewis acids are electron-pair acceptors. Lewis bases are electron-pair donors.
· All Arrhenius acids and bases are Brønsted–Lowry acids and bases, and all Brønsted–Lowry acids and bases are Lewis acids and bases; however, the converse of these statements is not necessarily true (that is, not all Lewis acids and bases are Brønsted–Lowry acids and bases, and not all Brønsted–Lowry acids and bases are Arrhenius acids and bases).
· Amphoteric species are those that can behave as an acid or base. Amphiprotic species are amphoteric species that specifically can behave as a Brønsted–Lowry acid or Brønsted–Lowry base.
o Water is a classic example of an amphoteric, amphiprotic species—it can accept a hydrogen ion to become a hydronium ion, or it can donate a hydrogen ion to become a hydroxide ion.
o Conjugate species of polyvalent acids and bases can also behave as amphoteric and amphiprotic species.
Properties
· The water dissociation constant, Kw, is 10−14 at 298 K. Like other equilibrium constants, Kw is only affected by changes in temperature.
· pH and pOH can be calculated given the concentrations of H3O+ and OH− ions, respectively. In aqueous solutions, pH + pOH = 14 at 298 K.
· Strong acids and bases completely dissociate in solution.
· Weak acids and bases do not completely dissociate in solution and have corresponding dissociation constants (Ka and Kb, respectively).
· In the Brønsted–Lowry theory, acids have conjugate bases that are formed when the acid is deprotonated. Bases have conjugate acids that are formed when the base is protonated.
o Strong acids and bases have very weak (inert) conjugates.
o Weak acids and bases have weak conjugates.
· Neutralization reactions form salts and (sometimes) water.
Polyvalence and Normality
· An equivalent is defined as one mole of the species of interest.
· In acid–base chemistry, normality is the concentration of acid or base equivalents in solution.
· Polyvalent acids and bases are those that can donate or accept multiple electrons. The normality of a solution containing a polyvalent species is the molarity of the acid or base times the number of protons it can donate or accept.
Titration and Buffers
· Titrations are used to determine the concentration of a known reactant in a solution.
o The titrant has a known concentration and is added slowly to the titrand to reach the equivalence point.
o The titrand has an unknown concentration but a known volume.
· The half-equivalence point is the midpoint of the buffering region, in which half of the titrant has been protonated (or deprotonated); thus, [HA] = [A−] and a buffer is formed.
· The equivalence point is indicated by the steepest slope in a titration curve; it is reached when the number of acid equivalents in the original solution equals the number of base equivalents added, or vice-versa.
o Strong acid and strong base titrations have equivalence points at pH = 7.
o Weak acid and strong base titrations have equivalence points at pH > 7.
o Weak base and strong acid titrations have equivalence points at pH < 7.
o Weak acid and weak base titrations can have equivalence points above or below 7, depending on the relative strength of the acid and base.
· Indicators are weak acids or bases that display different colors in their protonated and deprotonated forms.
o The indicator chosen for a titration should have a pKa close to the pH of the expected equivalence point.
o The endpoint of a titration is when the indicator reaches its final color.
· Multiple buffering regions and equivalence points are observed in polyvalent acid and base titrations.
· Buffer solutions consist of a mixture of a weak acid and its conjugate salt or a weak base and its conjugate salt; they resist large fluctuations in pH.
· Buffering capacity refers to the ability of a buffer to resist changes in pH; maximal buffering capacity is seen within 1 pH point of the pKa of the acid in the buffer solution.
· The Henderson–Hasselbalch equation quantifies the relationship between pH and pKa for weak acids and between pOH and pKb for weak bases; when a solution is optimally buffered, pH = pKa and pOH = pKb.
Answers to Concept Checks
· 10.1
1.
|
Definition |
Acid |
Base |
|
Arrhenius |
Dissociates to form excess H+ in solution |
Dissociates to form excess OH− in solution |
|
Brønsted–Lowry |
H+ donor |
H+ acceptor |
|
Lewis |
Electron pair acceptor |
Electron pair donor |
2.
|
Anion |
Acid Formula |
Acid Name |
|
MnO4− |
HMnO4 |
Permanganic acid |
|
Titanate (TiO32−) |
H2TiO3 |
Titanic acid |
|
I− |
HI |
Hydroiodic acid |
|
IO4− |
HIO4 |
Periodic acid |
3.
|
Reaction |
Amphoteric Reactant |
Amphiprotic? |
|
HCO3− + HBr → H2CO3 + Br− |
HCO3− |
Yes |
|
3 HCl + Al(OH)3 → AlCl3 + 3 H2O |
Al(OH)3 |
No |
|
2 HBr + ZnO → ZnBr2 + H2O |
ZnO |
No |
· 10.2
1. An amphoteric species can act as an acid or a base.
2. High Ka indicates a strong acid, which will dissociate completely in solution. Having a Ka slightly greater than water means the acid is a weak acid with minimal dissociation.
3. High Kb indicates a strong base, which will dissociate completely in solution. Having a Kb slightly greater than water means the base is a weak base with minimal dissociation.
4. 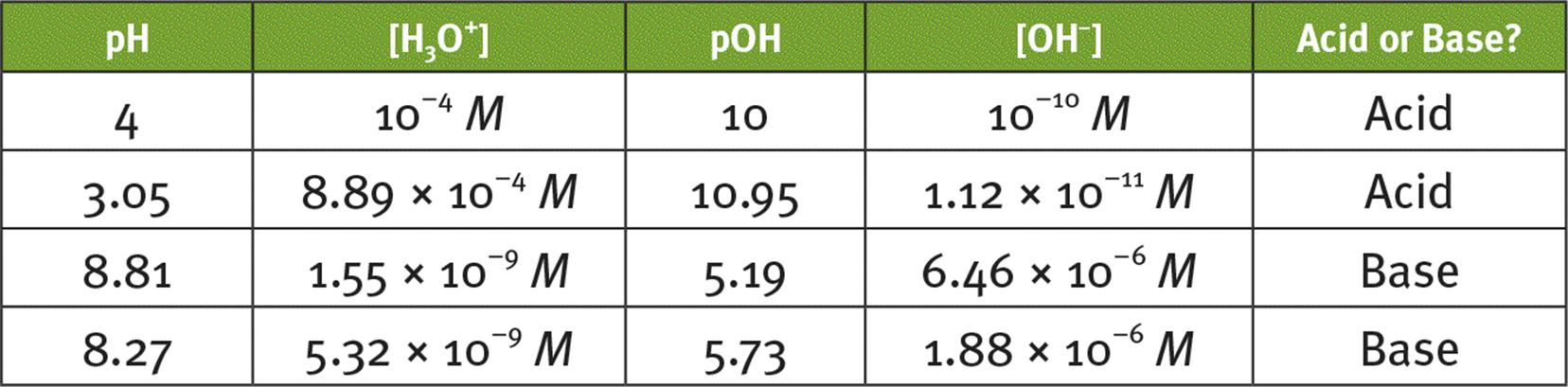
(Note: exact answers are provided; your rounded answers should be relatively close to those listed here.)
5. Ka × Kb = Kw
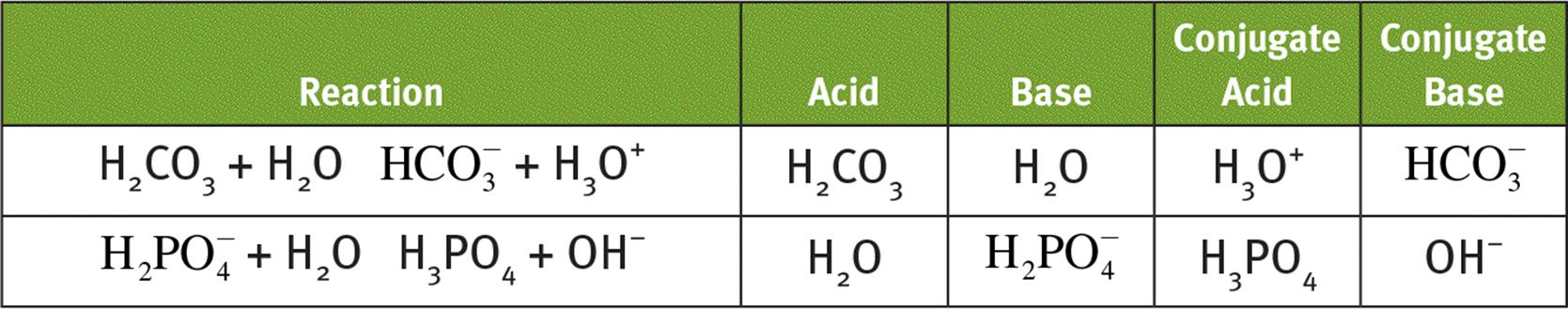
6. 
7. 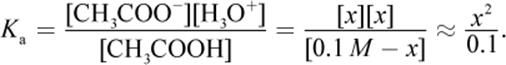
Therefore, x2 = 1.8 × 10−6 → x ≈ 1.3 × 10−3 M (actual = 1.35 × 10−3 M). Then, pH = −log H3O+ ≈ 3 − 0.13 = 2.87 (actual = 2.88)
· 10.3
1. Acids use moles of H+ (H3O+) as an equivalent. Bases use moles of OH− as an equivalent.
2. 6 N Al(OH)3; 32 N H2SO4
· 10.4
1. The buffering region occurs when [HA] ≈ [A−] and is the flattest portion of the titration curve (resistant to changes in pH). The half-equivalence point is the center of the buffering region, where [HA] = [A−]. The equivalence point is the steepest point of the titration curve, and occurs when the equivalents of acid present equal the equivalents of base added (or vice-versa). The endpoint is the pH at which an indicator turns its final color.
2. Phenolphthalein would be the preferred indicator for this titration.
3. A strong acid and weak base have an equivalence point in the acidic range. A strong base and weak acid have an equivalence point in the basic range. A strong acid and strong base have an equivalence point at pH = 7 (neutral). A weak acid and weak base can have an equivalence point in the acidic, neutral, or basic range, depending on the relative strengths of the acid and base.
4. A buffer solution is designed to resist changes in pH and has optimal buffering capacity within 1 pH point from its pKa.
5. 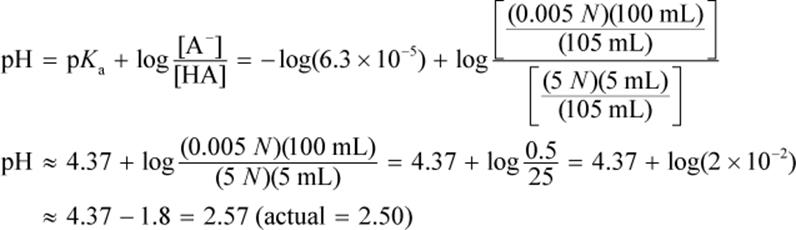
Equations to Remember
(10.1) Autoionization constant for water: Kw = [H3O+][OH−] = 10−14 at 25°C (298 K)
(10.2) Definitions of pH and pOH:
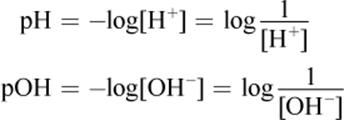
(10.3) Relationship of pH and pOH at 298 K: pH + pOH = 14
(10.4) p scale value approximation: p value ≈ m − 0.n
(10.5) Acid dissociation constant:
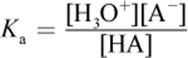
(10.6) Base dissociation constant:
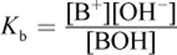
(10.7) Relationship of Ka and Kb at 298 K:

(10.8) Equivalence point: NaVa = NbVb
(10.9) Henderson–Hasselbalch equation (acid buffer):
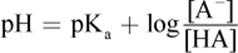
(10.10) Henderson–Hasselbalch equation (base buffer):
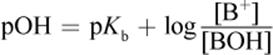
Shared Concepts
· Biology Chapter 6
o The Respiratory System
· Biology Chapter 10
o Homeostasis
· General Chemistry Chapter 3
o Bonding and Chemical Interactions
· General Chemistry Chapter 9
o Solutions
· Organic Chemistry Chapter 4
o Analyzing Organic Reactions
· Physics and Math Chapter 10
o Mathematics