MCAT General Chemistry Review
Chapter 1: Atomic Structure
Answers and Explanations
1. ARemember that when electrons are removed from an element, forming a cation, they will be removed from the subshell with the highest n value first. Zn0 has 30 electrons, so it would have an electron configuration of 1s22s22p63s23p64s23d10. The 4s subshell has the highest principal quantum number, so it is emptied first, forming 1s22s22p63s23p64s03d10. Choice (B) implies that electrons are pulled out of the d orbital, choice (C) presents the configuration of the uncharged zinc atom, and choice (D) shows the configuration that would exist if four electrons were removed.
2. BThe azimuthal quantum number l cannot be higher than n – 1, ruling out choice (A). The ml number, which describes the chemical’s magnetic properties, can only be an integer value between –l and l. It cannot be equal to 1 if l = 0; this would imply that an s orbital has three subshells (−1, 0, and 1) when we know it can only have one. This rules out choices (C) and (D).
3. CFor any value of n there will be a maximum of 2n2 electrons; that is, two per orbital. This can also be determined from the Periodic Table. There are only two elements (H and He) that have valence electrons in the n = 1 shell. Eight elements (Li to Ne) have valence electrons in the n= 2 shell. This is the only equation that matches this pattern.
4. B
This formula describes the number of electrons in terms of the azimuthal quantum number l, which ranges from 0 to n – 1, with n being the principal quantum number. A table of the maximum number of electrons per subshell is provided here:
|
Subshell |
Azimuthal Quantum Number (l) |
Number of Electrons |
|
s |
0 |
2 |
|
p |
1 |
6 |
|
d |
2 |
10 |
|
f |
3 |
14 |
5. DThe only answer choice without unpaired electrons in its ground state is helium. Recall from the chapter that a diamagnetic substance is identified by the lack of unpaired electrons in its shell. A substance without unpaired electrons, like helium, cannot be magnetized by an external magnetic field and is actually slightly repelled. Elements that come at the end of a block (Group IIA, the group containing Zn, and the noble gases, most notably) have only paired electrons.
6. D
While daunting at first, the problem requires the MCAT favorite equation ![]() where h = 6.626 × 10−34 J·s (Planck’s constant),
where h = 6.626 × 10−34 J·s (Planck’s constant), ![]() is the speed of light, and λ is the wavelength of the light. This question asks for the energy of one mole of photons, so we must multiply by Avogadro’s number, NA = 6.02 × 1023 mol−1.
is the speed of light, and λ is the wavelength of the light. This question asks for the energy of one mole of photons, so we must multiply by Avogadro’s number, NA = 6.02 × 1023 mol−1.
The setup is: ![]()
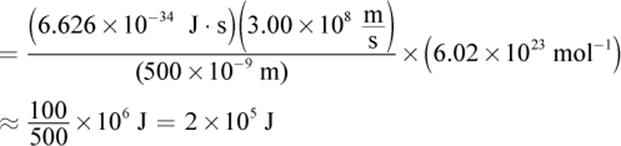
7. BBecause the electron is moving into the n = 1 shell, the only subshell available is the 1s subshell, which eliminates choices (C) and (D). There will be some energy change, however, as the electron must lose energy to return to the minimum-energy ground state. That will require emitting radiation in the form of a photon.
8. ARecall that the superscript refers to the mass number of an atom, which is equal to the number of protons plus the number of neutrons present in an element. Sometimes a text will list the atomic number, Z, as a subscript under the mass number, A. According to the Periodic Table, carbon contains six protons; therefore, its atomic number is 6. Isotopes all have the same number of protons, but differ in number of neutrons. Almost all atoms with Z > 1 have at least one neutron. Carbon is most likely to have a mass number of 12, for six protons and six neutrons, as in choice (B). Choices (C) and (D) are possible isotopes that would have more neutrons than 12C. The 6C isotope is unlikely. It would mean that there are 6 protons and 0 neutrons. As shown in Figure 1.4, this would be a highly unstable isotope.
9. CThe limitations placed by the Heisenberg uncertainty principle are caused by limitations inherent in the measuring process: if a particle is moving, it has momentum, but trying to measure that momentum necessarily creates uncertainty in the position. Even if we had an exact definition of the meter, as in choice (A), or perfect measuring devices, as in choice (B), we still wouldn’t be able to measure position and momentum simultaneously and exactly.
10.BFor the electron to gain energy, it must absorb energy from photons to jump up to a higher energy level. There is a bigger jump between n = 2 and n = 6 than there is between n = 3 and n = 4.
11.AThe MCAT covers the topics in this chapter qualitatively more often than quantitatively. It is critical to be able to distinguish the fundamental principles that determine electron organization, which are usually known by the names of the scientists who discovered or postulated them. The Heisenberg uncertainty principle, choice (B), refers to the inability to know the momentum and position of a single electron simultaneously. The Bohr model, choice (C), was an early attempt to describe the behavior of the single electron in a hydrogen atom. The Rutherford model, choice (D), described a dense, positively charged nucleus. The element shown here, nitrogen, is often used to demonstrate Hund’s rule because it is the smallest element with a half-filled p subshell. Hund’s rule explains that electrons fill empty orbitals first before doubling up electrons in the same orbital.
12.AThe quickest way to solve this problem is to use the Periodic Table and find out how many protons are in Cs atoms; there are 55. Neutral Cs atoms would also have 55 electrons. A stable Cs cation will have a single positive charge because it has one unpaired s-electron. This translates to one fewer electron than the number of protons, or 54 electrons.
13.B
The easiest way to approach this problem is to set up a system of two algebraic equations, where H and D are the percentages of H (mass = 1 amu) and D (mass = 2 amu), respectively. Your setup should look like the following system:
H + D = 1 (percent H + percent D = 100%)
1 H + 2 D = 1.008 (atomic weight calculation)
Rearranging the first equation and substituting into the second yields (1 – D) + 2D = 1.008, or D = 0.008. 0.008 is 0.8%, so there is 0.8% D.
14.AThe terms in the answer choices refer to the magnetic spin of the two electrons. The quantum number ms represents this property as a measure of an electron’s intrinsic spin. These electrons’ spins are parallel, in that their spins are aligned in the same direction (![]() for both species).
for both species).
15.B
When dealing with ions, you cannot directly approach electronic configurations based on the number of electrons they currently hold. First examine the neutral atom’s configuration, and then determine which electrons are removed.
|
Neutral Atom’s Configuration |
Ion’s Configuration |
|
Cr0: [Ar] 4s13d5 |
— |
|
Mn0: [Ar] 4s23d5 |
Mn+: [Ar] 4s13d5 |
|
Fe0: [Ar] 4s23d6 |
Fe2+: [Ar] 4s03d6 |
Due to the stability of half-filled d-orbitals, neutral chromium assumes the electron configuration of [Ar] 4s13d5. Mn must lose one electron from its initial configuration to become the Mn+ cation. That electron would come from the 4s subshell, according to the rule that the first electron removed comes from the highest-energy shell. Fe must lose two electrons to become Fe2+. They’ll both be lost from the same orbital; the only way Fe2+ could hold the configuration in the question stem would be if one d-electron and one s-electron were lost together.