MCAT General Chemistry Review
Chapter 11: Oxidation–Reduction Reactions
Conclusion
In this chapter, we covered the essential MCAT topic of oxidation–reduction reactions. We reviewed the rules for assigning oxidation numbers to help us keep track of the movement of electrons from the species that are oxidized (reducing agents) to the species that are reduced (oxidizing agents). We also covered the sequence of steps involved in balancing half-reactions, redox titrations, and disproportionation reactions.
In addition to understanding the fundamental chemical principles behind these reactions, you will begin to see these concepts resurface in MCAT Organic Chemistry Review and MCAT Biochemistry Review. Oxidation–reduction reactions are often used for energy transfer in biological systems, and any deficiencies in such systems are profoundly deleterious (such as metabolic, mitochondrial, and immunologic diseases). Our next chapter—the last of MCAT General Chemistry Review—brings the principles of oxidation–reduction reactions to their application in electrochemical cells. By the end of the next chapter, you will have reviewed all of the general chemistry knowledge required for Test Day!
Concept Summary
Oxidation–Reduction Reactions
· Oxidation is a loss of electrons, and reduction is a gain of electrons; the two are paired together in what is known as an oxidation–reduction (redox) reaction.
· An oxidizing agent facilitates the oxidation of another compound and is reduced itself in the process; a reducing agent facilitates the reduction of another compound and is itself oxidized in the process.
o Common oxidizing agents almost all contain oxygen or a similarly electronegative element.
o Common reducing agents often contain metal ions or hydrides (H–).
· To assign oxidation numbers, one must know the common oxidation states of the representative elements.
o Any free element or diatomic species has an oxidation number of zero.
o The oxidation number of a monatomic ion is equal to the charge of the ion.
o When in compounds, Group IA metals have an oxidation number of +1; Group IIA metals have an oxidation number of +2.
o When in compounds, Group VIIA elements have an oxidation number of –1 (unless combined with an element with higher electronegativity).
o The oxidation state of hydrogen is +1 unless it is paired with a less electronegative element, in which case it is –1.
o The oxidation state of oxygen is usually –2, except in peroxides (when its charge is –1) or in compounds with more electronegative elements.
o The sum of the oxidation numbers of all the atoms present in a compound is equal to the overall charge of that compound.
· When balancing redox reactions, the half-reaction method, also called the ion–electron method, is the most common.
o Separate the two half-reactions.
o Balance the atoms of each half-reaction. Start with all the elements besides H and O. In acidic solution, balance H and O using water and H+. In basic solution, balance H and O using water and OH–.
o Balance the charges of each half-reaction by adding electrons as necessary to one side of the reaction.
o Multiply the half-reactions as necessary to obtain the same number of electrons in both half-reactions.
o Add the half-reactions, canceling out terms on both sides of the reaction arrow.
o Confirm that the mass and charge are balanced.
Net Ionic Equations
· A complete ionic equation accounts for all of the ions present in a reaction. To write a complete ionic reaction, split all aqueous compounds into their relevant ions. Keep solid salts intact.
· Net ionic equations ignore spectator ions to focus only on the species that actually participate in the reaction. To obtain a net ionic reaction, subtract the ions appearing on both sides of the reaction, which are called spectator ions.
o For reactions that contain no aqueous salts, the net ionic equation is generally the same as the overall balanced reaction.
o For double displacement (metathesis) reactions that do not form a solid salt, there is no net ionic reaction because all ions remain in solution and do not change oxidation number.
· Disproportionation (dismutation) reactions are a type of redox reaction in which one element is both oxidized and reduced, forming at least two molecules containing the element with different oxidation states.
· Oxidation–reduction titrations are similar in methodology to acid–base titrations. These titrations follow transfer of charge.
o Indicators used in such titrations change color when certain voltages of solutions are achieved.
o Potentiometric titration is a form of redox titration in which a voltmeter or external cell measures the electromotive force (emf) of a solution. No indicator is used, and the equivalence point is determined by a sharp change in voltage.
Answers to Concept Checks
· 11.1
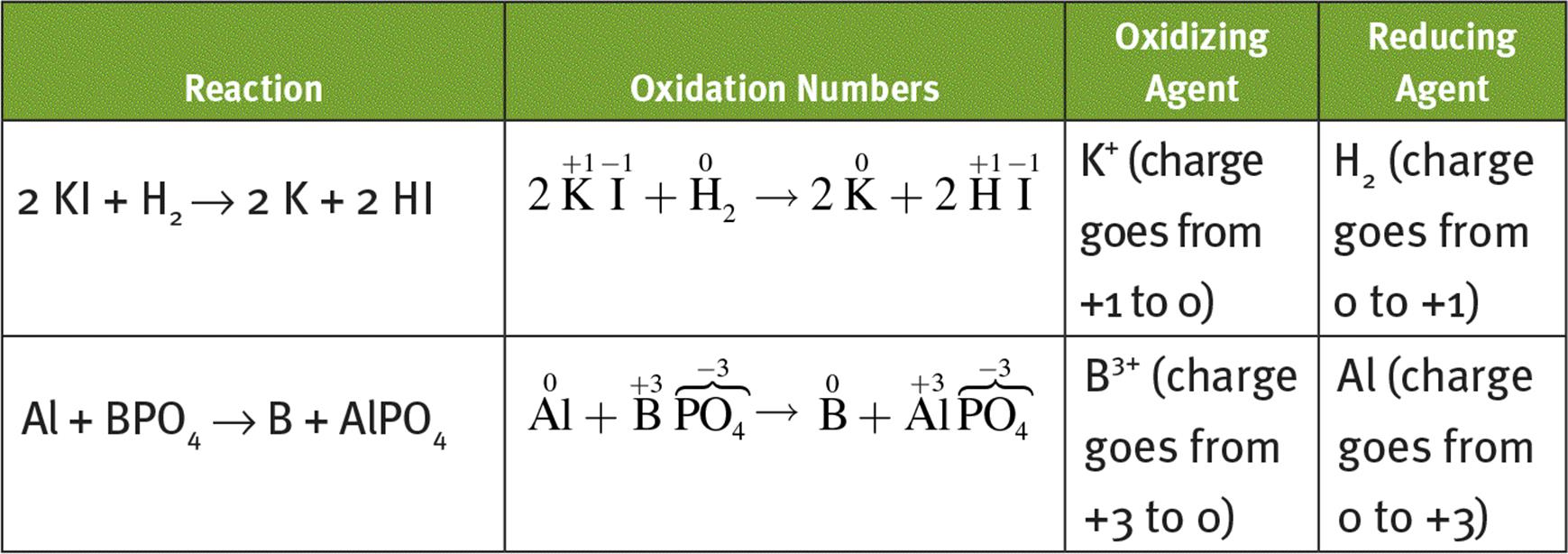
1. 
2. Oxidation: Zn → Zn2+ + 2 e–
Reduction: Cu2+ + 2 e– → Cu
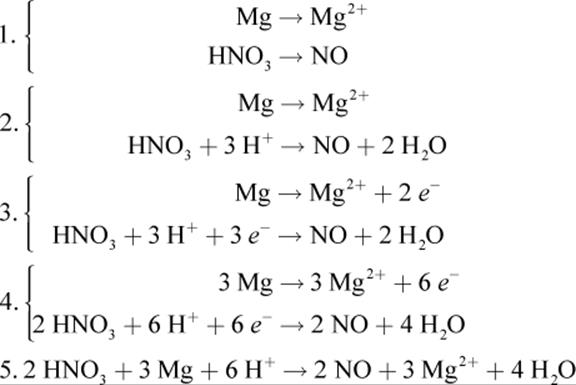
3. 
· 11.2
1. Cu+ + Cl– → CuCl
3 Mg + 2 Al3+ → 3 Mg2+ + 2 Al (don’t forget to balance the reaction!)
2. In the first reaction, chlorine undergoes disproportionation to have a –1 oxidation state in NaCl and a +5 oxidation state in NaClO3.
In the second reaction, sulfur undergoes disproportionation to have a 0 oxidation state in elemental sulfur and +4 oxidation state in SO2.
3. 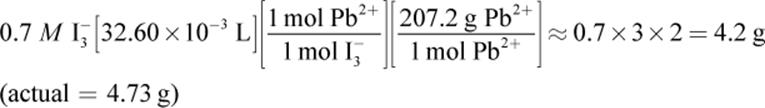
Shared Concepts
· Biochemistry Chapter 2
o Enzymes
· Biochemistry Chapter 10
o Carbohydrate Metabolism II
· Biology Chapter 7
o The Cardiovascular System
· General Chemistry Chapter 10
o Acids and Bases
· General Chemistry Chapter 12
o Electrochemistry
· Organic Chemistry Chapter 4
o Analyzing Organic Reactions