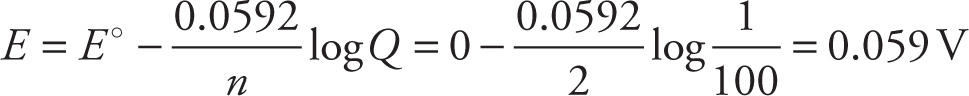MCAT General Chemistry Review - Steven A. Leduc 2015
Electrochemistry
12.1 OXIDATION-REDUCTION REACTIONS
Recall that the oxidation number (or oxidation state) of each atom in a molecule describes how many electrons it is donating or accepting in the overall bonding of the molecule. Many elements can assume different oxidation states depending on the bonds they make. A reaction in which the oxidation numbers of any of the reactants change is called an oxidation-reduction (or redox) reaction.
In a redox reaction, atoms gain or lose electrons as new bonds are formed. The total number of electrons does not change, of course; they’re just redistributed among the atoms. When an atom loses electrons, its oxidation number increases; this is oxidation. When an atom gains electrons, the oxidation number decreases; this is reduction. A mnemonic device is helpful:
LEO the lion says GER
LEO: Lose Electrons = Oxidation
GER: Gain Electrons = Reduction
Another popular mnemonic is
OIL RIG
OIL: Oxidation Is electron Loss
RIG: Reduction Is electron Gain
An atom that is oxidized in a reaction loses electrons to another atom. We call the oxidized atom a reducing agent or reductant, because by giving up electrons, it reduces another atom that gains the electrons. On the other hand, the atom that gains the electrons has been reduced. We call the reduced atom an oxidizing agent or oxidant, because it oxidizes another atom that loses the electrons. (You may want to review Section 3.13 on Oxidation States.)
Example 12-1: In an oxidation-reduction, the oxidation number of an aluminum atom changes from 0 to +3. The aluminum atom has been:
A) reduced, and is a reducing agent.
B) reduced, and is an oxidizing agent.
C) oxidized, and is a reducing agent.
D) oxidized, and is an oxidizing agent.
Solution: Since the oxidation number has increased, the atom’s been oxidized. And since it’s been oxidized, it reduced something else and thus acted as a reducing agent. Therefore, the answer is C.
Take a look at this redox reaction:
Fe + 2 HCl → FeCl2 + H2
The oxidation state of iron changes from 0 to +2. The oxidation state of hydrogen changes from +1 to 0. (The oxidation state of chlorine remains at —1.) So, iron has lost two electrons, and two protons (H+) have gained one electron each. Therefore, the iron has been oxidized, and the hydrogens have been reduced. In order to better see the exchange of electrons, a redox reaction can be broken down into a pair of half-reactions that show the oxidation and reduction separately. These ion-electron equations show only the actual oxidized or reduced species—and the electrons involved—in an electron-balanced reaction. For the redox reaction shown above, the ion-electron half-reactions are:
oxidation: |
Fe → Fe2+ + 2e− |
reduction: |
2 H+ + 2e− → H2 |
Example 12-2: For the redox reaction
3 MnO2 + 2 Al → 2 Al 2O3 + 3 Mn
which of the following shows the oxidation half-reaction?
A) Mn4+ + 4e− → Mn
B) Mn2+ + 2e− → Mn
C) Al → Al3+ + 3e−
D) Al4+ → Al6+ + 6e−
Solution: First, eliminate choices A and B since they’re reduction half-reactions. We can then eliminate choice D for two reasons: First, Al4+ is not the species of aluminum on the reactant side; second, it’s not balanced electrically. The answer must be C.
12.2 GALVANIC CELLS
Because a redox reaction involves the transfer of electrons, and the flow of electrons constitutes an electric current that can do work, we can use a spontaneous redox reaction to generate an electric current. A device for doing this is called a galvanic (or voltaic) cell, the main features of which are shown in the figure below.
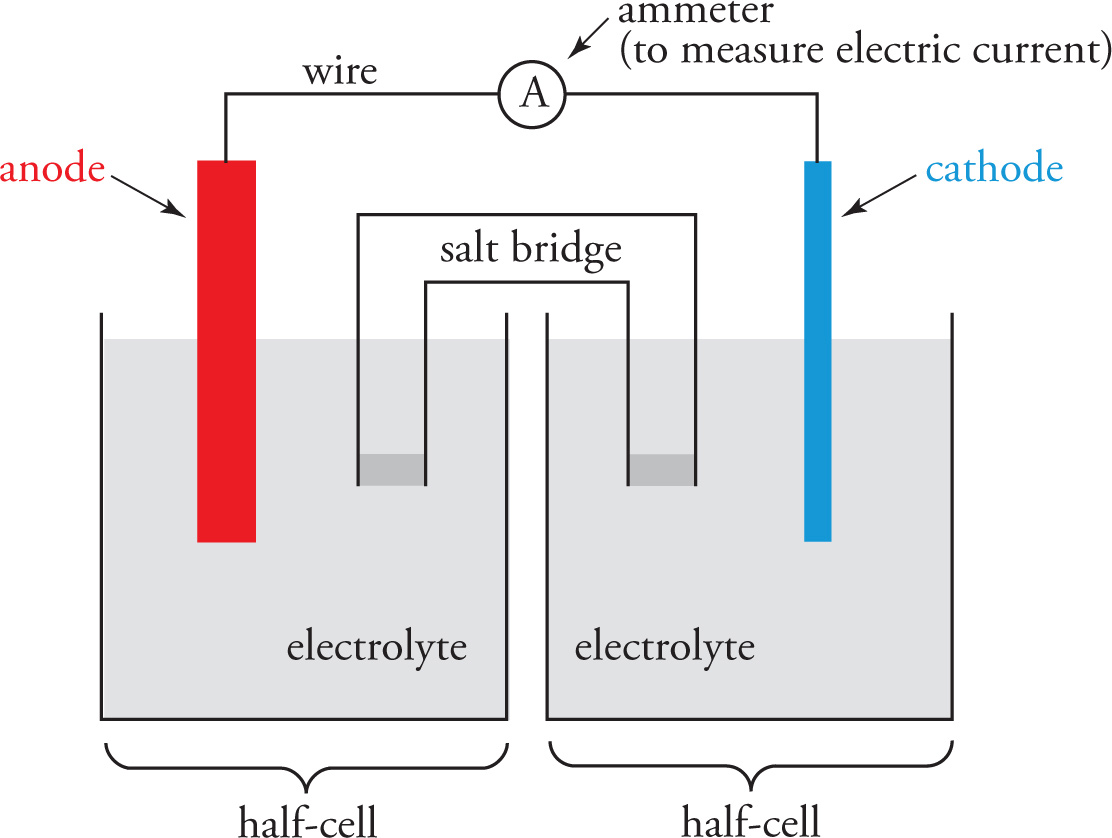
One electrode, generally composed of a metal (labeled the anode) gets oxidized, and the electrons its atoms lose travel along the wire to a second metal electrode (labeled the cathode). It is at the cathode that reduction takes place. In this way, the anode acts as an electron source, while the cathode acts as an electron sink. Electrons always flow through the conducting wire from the anode to the cathode. This electron flow is the electric current that is produced by the spontaneous redox reaction between the electrodes.
Let’s look at a specific galvanic cell, with the anode made of zinc, and the cathode made of copper. The anode is immersed in a ZnSO4 solution, the cathode is immersed in a CuSO4 solution, and the half cells are connected by a salt bridge containing an aqueous solution of KNO3. When the electrodes are connected by a wire, zinc atoms in the anode are oxidized (Zn → Zn2+ + 2e−), and the electrons travel through the wire to the cathode. There, the Cu2+ ions in solution pick up these electrons and get reduced to copper metal (Cu2+ + 2e− → Cu), which accumulates on the copper cathode. The sulfate anions balance the charge on the Zn2+, but do not participate in any redox reaction, and are therefore known as spectator ions.
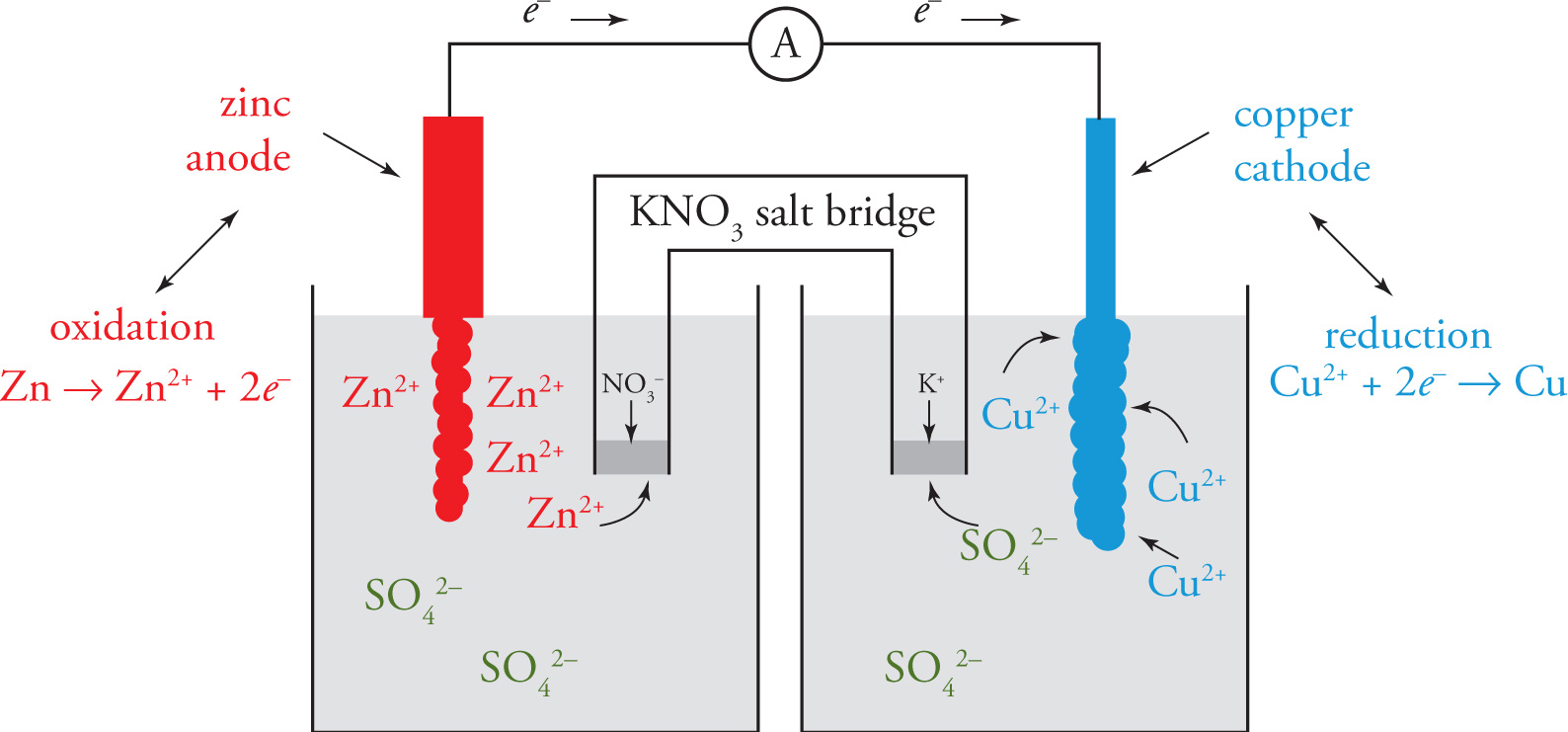
The Zn2+ ions that remain in the solution in the zinc half-cell attract NO3− ions from the salt bridge, and K+ ions in the salt bridge are attracted into the copper half-cell. Notice that anions in solution travel from the right cell to the left cell—and cations travel in the opposite direction—using the salt bridge as a conduit. This movement of ions completes the circuit and allows the current in the wire to continue, until the zinc strip is consumed. Remember that anions from the salt bridge go to the anode and cations from the salt bridge go to the cathode.
Notice that the anode is always the site of oxidation, and the cathode is always the site of reduction. One way to help remember this is just to notice that “a” and “o” (anode and oxidation) are both vowels, while “c” and “r” (cathode and reduction) are both consonants. Another popular mnemonic is “an ox, red cat” for “anode = oxidation, reduction = cathode.”
We often use a shorthand notation, called a cell diagram, to identify the species present in a galvanic cell. Cell diagrams are read as follows:
Anode | Anodic solution (concentration) | | Cathodic solution (concentration) | Cathode
If the concentrations are not specified in the cell diagram, you should assume they are 1 M.
Example 12-3: In the electrochemical cell described by the following cell diagram, what reaction occurs at the anode?
Zn(s) | Zn2+(aq) | | Cl−(aq) | Cl2(g)
A) Zn → Zn2+ + 2e−
B) Zn2+ + 2e− → Zn
C) 2 Cl− → Cl2 + 2e−
D) Cl2 + 2e− → 2 Cl−
Solution: For any electrochemical cell, oxidation occurs at the anode, and reduction occurs at the cathode. Therefore, we’re looking for an oxidation, and choices B and D are eliminated. Since Zn is present at the anode, the answer is A.
Example 12-4: In the absence of a salt bridge, charge separation develops. The anode develops a positive charge and the cathode develops a negative charge, quickly halting the flow of electrons. In this state, the battery resembles:
A) a resistor.
B) a capacitor.
C) a transformer.
D) an inductor.
Solution: The question tells us that the result of removing a salt bridge is charge separation. In physics, we learned that a capacitor is a device that stores electrical energy due to the separation of charge on adjacent surfaces. Thus, choice B is the correct choice.
12.3 STANDARD REDUCTION POTENTIALS
To determine whether the redox reaction of a cell is spontaneous and can produce an electric current, we need to figure out the cell voltage. Each half-reaction has a potential (E), which is the cell voltage it would have if the other electrode were the standard reference electrode. (Note: We usually consider cells at standard conditions: 25°C, 1 atm pressure, aqueous solutions at 1 M concentrations, and with substances in their standard states. To indicate standard conditions, we use a ° superscript on quantities such as E and ∆G.) By definition, the standard reference electrode is the site of the redox reaction 2 H+ + 2e− → H2, which is assigned a potential of 0.00 volts. By adding the half-reaction potential for a given pair of electrodes, we get the cell’s overall voltage. Tables of half-reaction potentials are usually given for reductions only. Since each cell has a reduction half-reaction and an oxidation half-reaction, we get the potential of the oxidation by simply reversing the sign of the corresponding reduction potential.
For example, the standard reduction potential for the half-reaction Cu2+ + 2e− → Cu is +0.34 V. The standard reduction potential for the half-reaction Zn2+ + 2e− → Zn is —0.76 V. Reversing the zinc reduction to an oxidation, we get Zn → Zn2+ + 2e−, with a potential of +0.76 V. Therefore, the overall cell voltage for the zinc-copper cell is (+0.76 V) + (+0.34 V) = +1.10 V:
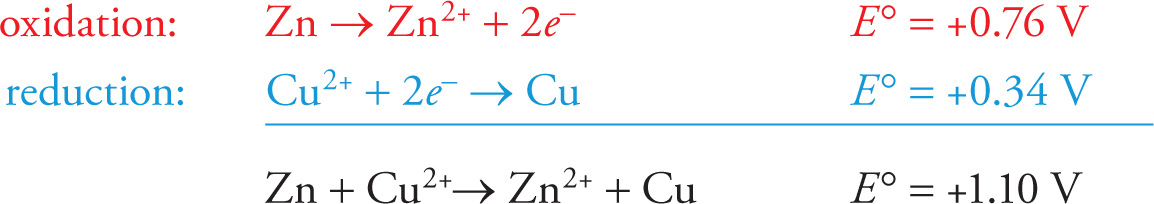
The free-energy change, ∆G°, for a redox reaction in which cell voltage is E° is given by the equation
∆G° = — nFE°
where n is the number of moles of electrons transferred and F stands for a faraday (the magnitude of the charge of one mole of electrons, approximately 96,500 coulombs). Since a reaction is spontaneous if ∆G° is negative, this equation tells us that the redox reaction in a cell will be spontaneous if the cell voltage is positive. Since the cell voltage for our zinc-copper cell was +1.10 V, we know the reaction will be spontaneous and produce an electric current that can do work.
If the cell voltage is positive, then the reaction is spontaneous.
If the cell voltage is negative, then the reaction is nonspontaneous.
Let’s do another example and consider what would happen if the zinc electrode in our cell above were replaced by a gold electrode, given that the standard reduction potential for the reaction Au3+ + 3e− → Au is E° = +1.50 V. The redox reaction that we’re investigating is
Au + Cu2+ → Au3+ + Cu
Let’s first break this down into half-reactions:
Au → Au3+ + 3e− |
E° = —1.50 V |
Cu2+ + 2e− → Cu |
E° = +0.34 V |
The overall reaction is not electron balanced; but by multiplying the first half-reaction by 2 and the second half-reaction by 3, we get
2 Au → 2 Au3+ + 6e− |
E° = —1.50 V |
3 Cu2+ + 6e− → 3 Cu |
E° = +0.34 V |
2 Au + 3 Cu2+ → 2 Au3+ + 3 Cu |
E° = —1.16 V |
The final equation is now electron balanced. Notice that although we multiplied the half-reactions by stoichiometric coefficients, we did not multiply the potentials by those coefficients. You never multiply the potential by a coefficient, even if you multiply a half-reaction by a coefficient to get the balanced equation for the reaction. This is because the potentials are intrinsic to the identities of the species involved and do not depend on the number of moles of the species.
Because the cell voltage is negative, this reaction would not be spontaneous. However, it would be spontaneous in the other direction; that is, if copper were the anode and gold the cathode, then the potential of the cell would be +1.16 V, which implies a spontaneous reaction.
Oxidizing and Reducing Agents
We can also use reduction potentials to determine whether reactants are good or poor oxidizing or reducing agents. For example, let’s look again at the half-reactions in our original zinc-copper cell. The half-reaction Zn2+ + 2e− → Zn has a standard potential of —0.76 V, and the half-reaction Cu2+ + 2e− → Cu has a standard potential of +0.34 V. The fact that the reduction of Zn2+ is nonspontaneous means that the oxidation of Zn is spontaneous, so Zn would rather give up electrons. If it does, this means that Zn acts as a reducing agent because in giving up electrons it reduces something else. The fact that the reduction of Cu2+ has a positive potential tells us that this reaction would be spontaneous at standard conditions. In other words, Cu2+ is a good oxidizing agent because it’s looking to accept electrons, thereby oxidizing something else. So, in general, if a reduction half-reaction has a large negative potential, then the product is a good reducing agent. On the other hand, if a reduction half-reaction has a large positive potential, then the reactant is a good oxidizing agent. Now, whether something is a “good” oxidizing or reducing agent depends on what it’s being compared to. So, to be more precise, we should say this:
The more negative the reduction potential, the weaker the reactant is as an oxidizing agent, and the stronger the product is as a reducing agent.
The more positive the reduction potential, the stronger the reactant is as an oxidizing agent, and the weaker the product is as a reducing agent.
For example, given that Pb2+ + 2e− → Pb has a standard potential of —0.13 V, and Al3+ + 3e− → Al has a standard potential of —1.67 V, what could we conclude? Well, since Al3+ has a large negative reduction potential, the product, aluminum metal, is a good reducing agent. In fact, because the reduction potential of Al3+ is more negative than that of Pb2+, we’d say that aluminum is a stronger reducing agent than lead.
Reaction |
E° (volts) |
Li+ + e → Li |
—3.05 |
Mg2+ + 2 e→ Mg |
—2.36 |
Al3+ + 3 e→ Al |
—1.67 |
Zn2+ + 2 e→ Zn |
—0.76 |
Fe2+ + 2 e→ Fe |
—0.44 |
Pb2+ + 2 e→ Pb |
—0.13 |
2 H+ + 2 e→ H2 |
0.00 |
Cu2+ + 2 e→ Cu |
0.34 |
Ag+ + e→ Ag |
0.80 |
Pd2+ + 2 e→ Pd |
0.99 |
Pt2+ + 2 e→ Pt |
1.20 |
Au3+ + 3 e→ Au |
1.50 |
F2 + 2 e→ 2 F− |
2.87 |
Example 12-5: A galvanic cell is set to operate at standard conditions. If one electrode is made of magnesium and the other is made of copper, then the magnesium electrode will serve as the:
A) anode and be the site of oxidation.
B) anode and be the site of reduction.
C) cathode and be the site of oxidation.
D) cathode and be the site of reduction.
Solution: First, eliminate choices B and C since the anode is always the site of oxidation and the cathode is always the site of reduction. From the table, we see that the reduction of Mg2+ is nonspontaneous, whereas the reduction of Cu2+ is spontaneous. Therefore, the copper electrode will serve as the cathode and be the site of reduction, and the magnesium electrode will serve as the anode and be the site of oxidation (choice A).
Example 12-6: What is the standard cell voltage for the reduction of Ag+ by Al?
Solution: The half-reactions are
Ag+ + e− → Ag |
E° = 0.80 V |
Al → Al3+ + 3e− |
E° = +1.67 V |
Although we’d multiply both sides of the first half-reaction by the stoichiometric coefficient 3 before adding it to the second one to give the overall, electron-balanced redox reaction, we don’t bother to do that here. The question is asking only for E°, and the potentials of the half-reactions are not affected by stoichiometric coefficients. Adding the potentials of these half-reactions gives us the overall cell voltage: E° = 2.47 V.
Example 12-7: For the reaction below, which of the following statements is true?
2 Au + 3 Fe2+ → 2 Au3+ + 3 Fe
A) The reaction is spontaneous, because its cell voltage is positive.
B) The reaction is spontaneous, because its cell voltage is negative.
C) The reaction is not spontaneous, because its cell voltage is positive.
D) The reaction is not spontaneous, because its cell voltage is negative.
Solution: First, eliminate choices B and C. Even without looking at the table of reduction potentials, these choices can’t be correct. If the cell voltage E° is negative, then the reaction is nonspontaneous, and if E° is positive, then the reaction is spontaneous. The half-reactions are
2 (Au → Au3+ + 3e−) |
E° = —1.50 V |
3 (Fe2+ + 2e− → Fe) |
E° = —0.44 V |
Notice again that the question is really asking only about E°, and the potentials of the half-reactions are not affected by stoichiometric coefficients (2 and 3, in this case). Since each of these half-reactions has a negative value for E°, the cell voltage (obtained by adding them) will also be negative, so the answer is D.
Example 12-8: Of the following, which is the strongest reducing agent?
A) Zn
B) Fe
C) Pd
D) Pd2+
Solution: Remember the rule: The more negative the reduction potential, the stronger the product is as a reducing agent. Zn (choice A) is the product of a redox half-reaction whose potential is —0.76 V. Fe (choice B) is the product of a redox half-reaction whose potential is —0.44 V. So, we know we can eliminate choice B. Pd (choice C) is the product of a redox half-reaction whose potential is +0.99 V, so C is eliminated. Finally, in order for Pd2+ to be a reducing agent, it would have to be oxidized—that is, lose more electrons. A cation getting further oxidized? Not likely, especially when there’s a neutral metal (choice A) that is happier to do so.
Example 12-9: Of the following, which is the strongest oxidizing agent?
A) Al3+
B) Ag+
C) Au3+
D) Cu2+
Solution: Remember the rule: The more positive the reduction potential, the stronger the reactant is as an oxidizing agent. Al3+ (choice A) is the reactant of a redox half-reaction whose potential is negative, so we can probably eliminate choice A right away. Ag+ (choice B) is the reactant of a redox half-reaction whose potential is +0.80 V. (Now we know that A can be eliminated). Au3+ (choice C) is the reactant of a redox half-reaction whose potential is +1.50 V, so now B is eliminated. Finally, Cu2+ (choice D) is the reactant of a redox half-reaction whose potential is only +0.34 V, so choice C is better.
Example 12-10: Which of the following best approximates the value of ∆G° for this reaction:
2 Al + 3 Cu2+ → 2 Al3+ + 3 Cu?
A) —(12)(96,500) J
B) —(6)(96,500) J
C) +(6)(96,500) J
D) +(12)(96,500) J
Solution: The half-reactions are
2 (Al → Al3+ + 3e−) |
E° = +1.67 V |
3 (Cu2+ + 2e− → Cu) |
E° = 0.34 V |
so the overall cell voltage is E° = 2.01 V ≈ 2 V. Because the number of electrons transferred is n = 2 × 3 = 6, the equation ∆G° = — nFE° tells us that choice A is the answer:
∆G° = — (6)(96,500)(2) J = — (12)(96,500) J
12.4 NONSTANDARD CONDITIONS
All the previous discussion of potentials assumed the conditions to be standard state, meaning that all aqueous reactants in the mixture were 1 M in concentration. So long as this is true, the tabulated values for reduction potentials apply to each half reaction.
However, since conditions are not always standard we must have a way to alternatively, and more generally, describe the voltage of an electrochemical reaction. To do this we use the Nernst equation.
Recall the following relationship:
∆G = ∆G° + RT ln Q
If we substitute ∆G and ∆G° with their respective relation to E and E°, we arrive at
—nFE = —nFE° + RT ln Q
or
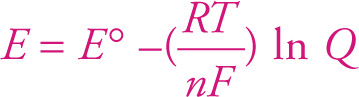
This is the Nernst equation. It describes how deviations in temperature and concentration of reactants can alter the voltage of a reaction under nonstandard conditions. As in the standard chemical systems previously discussed, the concentrations of product and reactants will change until Q = Keq, and E = 0.
Concentration Cells
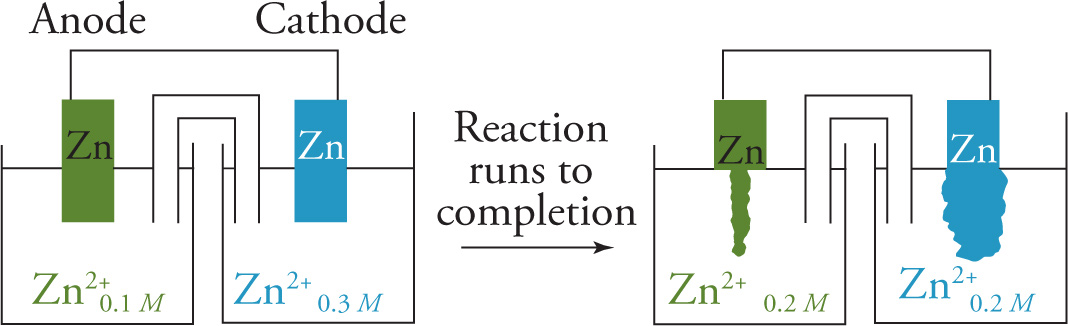
A concentration cell is a galvanic cell that has identical electrodes but which has half-cells with different ion concentrations. Since the electrodes and relevant ions in the two beakers have the same identities, the standard cell voltage, E°, would be zero. But, such a cell is not standard because both electrolytic solutions in the half-cells are not 1 M. So even though the electrodes are the same, in a concentration cell there will be a potential difference between them, and an electric current will be produced. For example, let’s say both electrodes are made of zinc, and the [Zn2+] concentrations in the electrolytes were 0.1 M and 0.3 M, respectively. We’d expect electrons to be induced to flow through the conducting wire to the half-cell with the higher concentration of these positive ions. So, the zinc electrode in the 0.1 M solution would serve as the anode, with the liberated electrons flowing across the wire to the zinc electrode in the 0.3 M solution, which serves as the cathode. When the concentrations of the solutions become equal, the reaction will stop.
12.5 REDOX TITRATIONS
Just as the titration of an acid with a strong base of known concentration can provide information about the initial acid solution (most notably the concentration and pKa), titration of a redox active species with a strong oxidant or reductant can be used to determine similar unknowns.
Most redox titrations involve the use of a redox indicator. Much like an indicator in acid/base chemistry, a redox indicator uses a change in color to determine the endpoint. However, in a redox reaction, this change in color is due to a change in oxidation state rather than loss or gain of a proton. One commonly used redox indicator is the Ce4+ ion, a strong oxidant according to the equation below:
Ce4+ + 1 e− → Ce3+ |
E° ≈ 1.5 V (1 M HCl solution) |
The Ce4+ ion is bright yellow in solution, whereas the reduced Ce3+ is colorless. This color change, along with the comparatively high redox potential, make Ce4+ an ideal indicator for the determination of the concentration of solutions of oxidizable species.
For example, cerium is known to oxidize secondary alcohols to ketones in aqueous solution. As such, titration with Ce4+ is an appropriate method for the determination of alcohol concentration in solution, or for the determination of the number of secondary hydroxyl groups present in a chemical species. As long as the Ce4+ added to the solution is consumed, the solution will remain colorless. However, the solution will turn yellow immediately after all oxidizable hydroxyls have been consumed. Knowledge of the concentration of the Ce4+ titrant allows for the determination of initial −OH concentration.
A redox titration curve, similar to an acid-base titration curve, can be plotted for any such redox titration. An example is given below where a generic reductant is titrated with Ce4+.
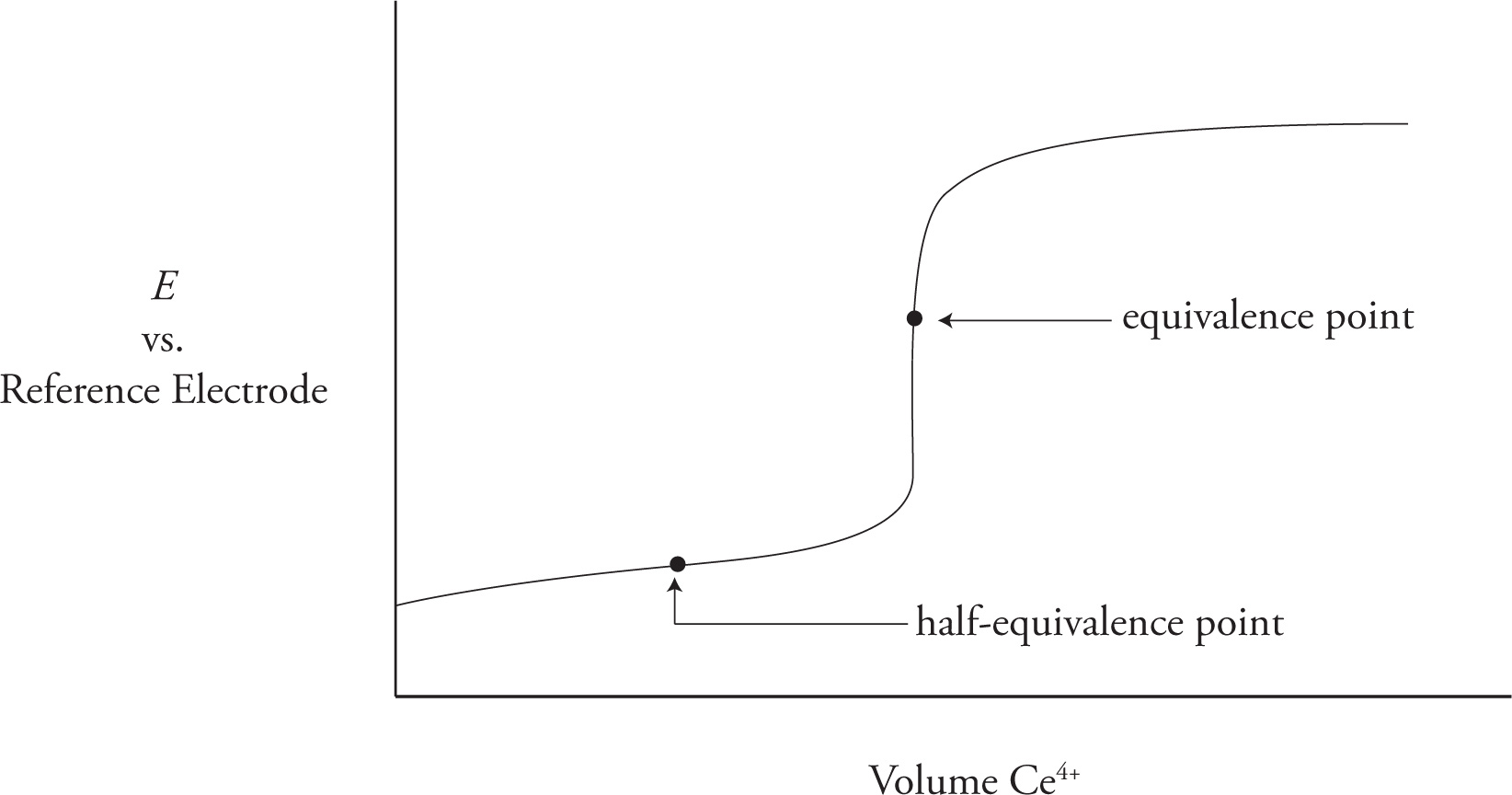
The equivalence point on the plot above will coincide with the solution turning yellow, indicating the completion of the redox reaction and the total consumption of the reductant as described by the system’s balanced redox equation. The significance of the half-equivalence point can be seen in the Nernst equation:
E = E° — (RT/nF) ln Q
In this case, Q refers to the ratio of oxidized and non-oxidized reactant. At the half-equivalence point these two quantities are equal and Q = 1. Since ln(1) = 0, at the half equivalence point the value of E (measured against whichever reference electrode one chooses) is equal to the value of E° for the redox couple being titrated.
12.6 ELECTROLYTIC CELLS
Unlike a galvanic cell, an electrolytic cell uses an external voltage source (such as a battery) to create an electric current that forces a nonspontaneous redox reaction to occur. This is known as electrolysis. A typical example of an electrolytic cell is one used to form sodium metal and chlorine gas from molten NaCl.
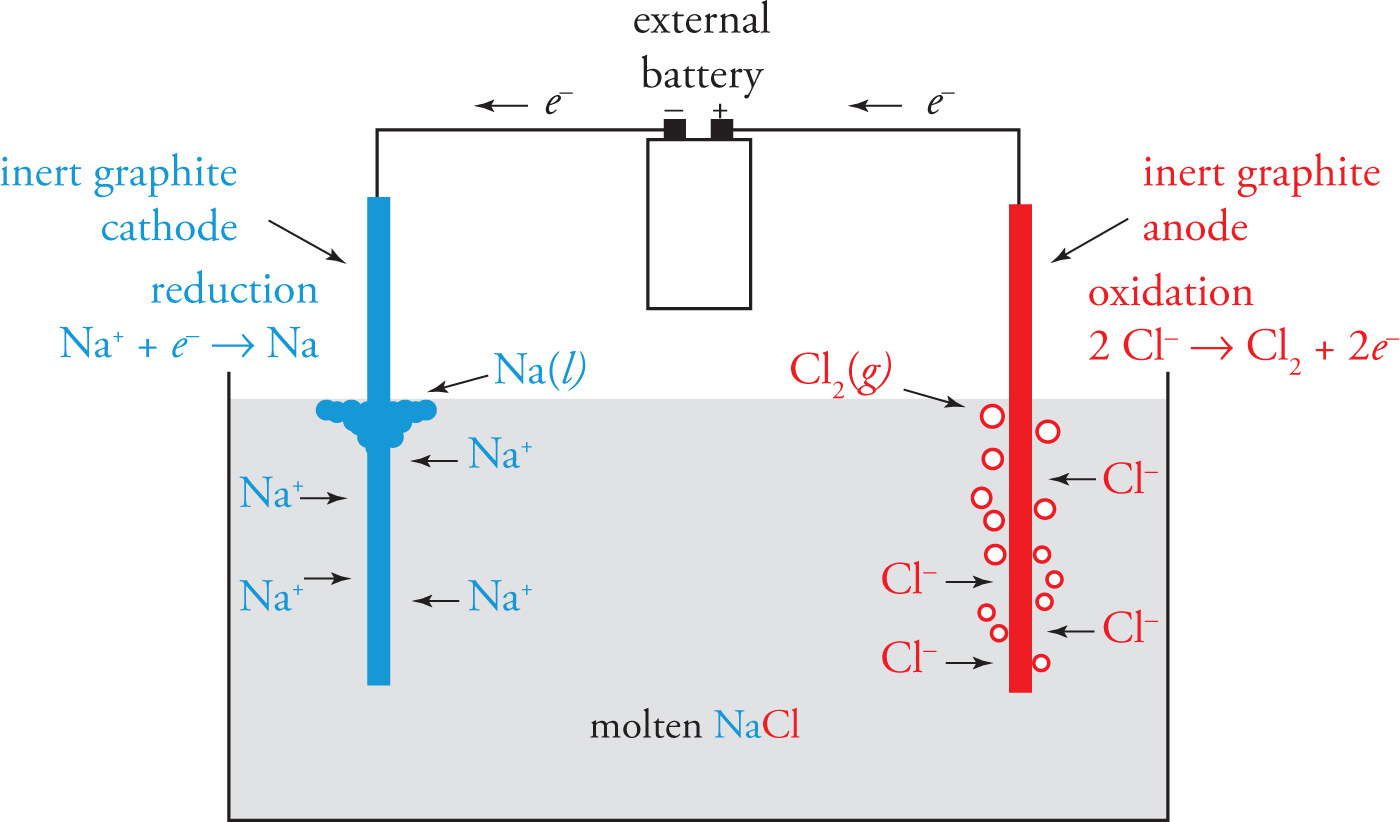
The half-reactions for converting molten Na+Cl− into sodium and chlorine are:
Na+ + e− → Na(l) |
E° = —2.71 V |
2 Cl− → Cl2(g) + 2e− |
E° = —1.36 V |
The standard voltage for the overall reaction is —4.07 V, which means the reaction is not spontaneous. The electrolytic cell shown above uses an external battery to remove electrons from chloride ions and forces sodium ions to accept them. In so doing, the sodium ions are reduced to sodium metal, and the chloride ions are oxidized to produce chlorine gas, which bubbles out of the electrolyte.
Electrolytic cells are also used for plating a thin layer of metal on top of another material, a process known as electroplating. If a fork is used as the cathode in an electrolytic cell whose electrolyte contains silver ions, silver will precipitate onto the fork, producing a silver-plated fork. Other examples of metal plating include gold-plated jewelry, and plating tin or chromium onto steel (for tin cans and car bumpers).
Galvanic vs. Electrolytic Cells
Notice that in both galvanic cells and electrolytic cells, the anode is the site of oxidation and the cathode is the site of reduction. Furthermore, electrons in the external circuit always move from the anode to the cathode. The difference, of course, is that a galvanic cell uses a spontaneous redox reaction to create an electric current, whereas an electrolytic cell uses an electric current to force a nonspontaneous redox reaction to occur.
It follows that in a galvanic cell, the anode is negative and the cathode is positive since electrons are spontaneously moving from a negative to a positive charge. However, in an electrolytic cell the anode is positive and the cathode is negative since electrons are being forced to move where they don’t want to go.
|
Galvanic |
Electrolytic |
|
Reduction at cathode |
|
Spontaneously generates electrical power (∆G° < 0) |
Nonspontaneous, requires an external electric power source (∆G° > 0) |
Total E° of reaction is positive |
Total E° of reaction is negative |
Anode is negative |
Anode is positive |
Cathode is positive |
Cathode is negative |
Example 12-11: The final products of the electrolysis of aqueous NaCl are most likely:
A) Na(s) and Cl2(g)
B) HOCl(aq) and Na(s)
C) Na(s) and O2(g)
D) NaOH(aq) and HOCl(aq)
Solution: This is a little tricky, but it provides a good example of using the process of elimination. We’re not expected to be able to answer this question outright, since there is virtually no information provided. Instead, realize that choices A, B and C list metallic sodium as a final product. That’s a problem because we’re in an aqueous medium, and we know that sodium metal reacts violently in water to form NaOH and hydrogen gas and fire. So after eliminating these choices, we’re left with choice D.
Common Rechargeable Batteries
One particularly useful galvanic cell uses two different oxidations states of Pb for its constitutive electrodes and sulfuric acid as an electrolyte. Often referred to as lead-acid batteries, these cells constitute the oldest type of rechargeable batteries, and are perhaps most commonly employed as automobile batteries.
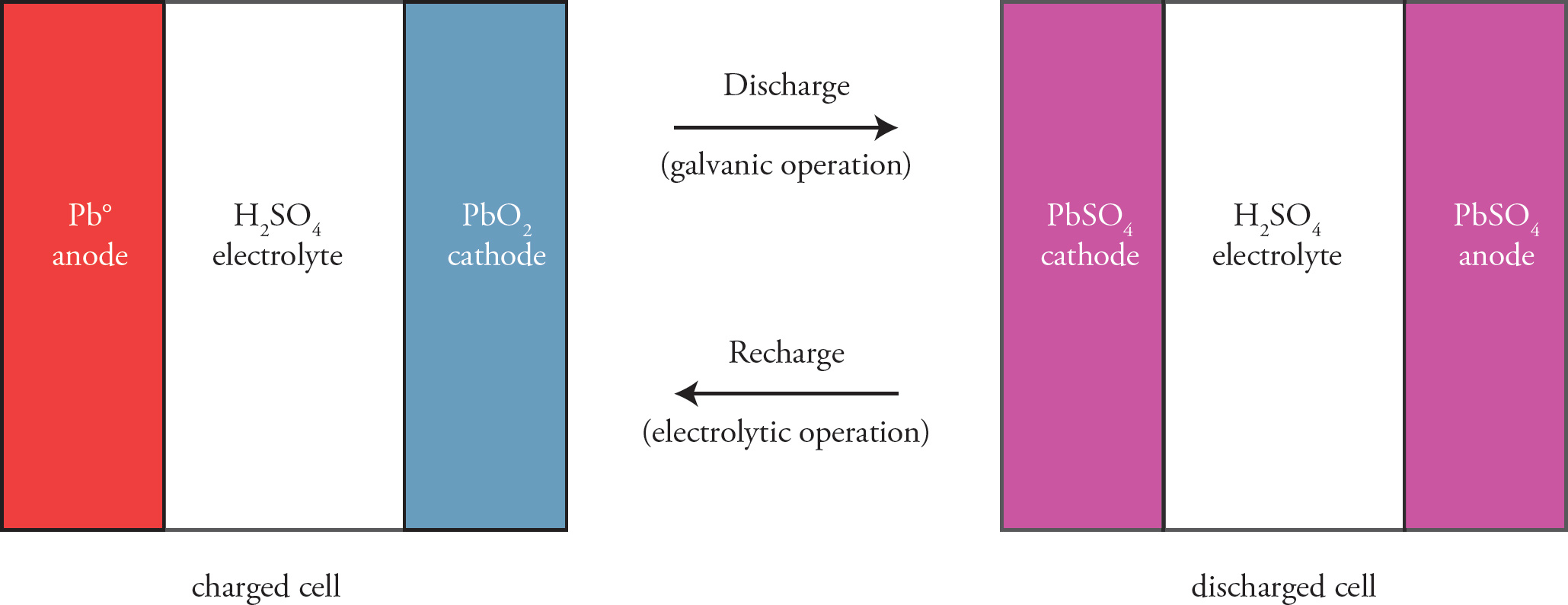
As depicted in the simplified figure above, fully charged lead acid batteries utilize Pb° as an anodic electrode and a cathode consisting of PbO2. As the battery discharges, Pbo undergoes a two-electron oxidation to PbSO4, while PbO2 is reduced to the same species, as described by the following equations.
Pb° + HSO4− → PbSO4 + H+ + 2 e−
PbO2 + HSO4− + 3 H+ + 2e− → PbSO4 + 2 H2O
Recharging the battery involves reversing the electron flow of discharge with applied voltage, as an electrolytic cell. The oxidation of PbSO4 back to PbO2, along with the regeneration of Pb° by the reduction of PbSO4 restores the initial potential of the cell.
Nickel-cadmium batteries, or NiCad batteries, are another common type of rechargeable battery. These cells utilize a metallic Cd° anode and a nickel oxide hydroxide (NiO(OH)) cathode. The redox reactions involved in the discharge of the battery are given below. To facilitate these reactions, NiCad cells contain an alkaline KOH electrolyte.
Cd° + 2 OH− → Cd(OH)2 + 2 e−
2 NiO(OH) + 2 H2O + 2 e− → 2 Ni(OH)2 + 2 OH−
Recharging spent NiCad batteries, as one might expect, involves applying a voltage to run these two reactions in reverse (typical electrolytic-cell behavior).
12.7 FARADAY’S LAW OF ELECTROLYSIS
We can determine the amounts of sodium metal and chlorine gas produced at the electrodes in the electrolytic cell shown in Section 12.6 using Faraday’s law of electrolysis:
Faraday’s Law of Electrolysis
The amount of chemical change is proportional to the amount of electricity that flows through the cell.
For example, let’s answer this question: If 5 amps of current flowed in the NaCl electrolytic cell for 1930 seconds, how much sodium metal and chlorine gas would be produced?
Step 1: First determine the amount of electricity (in coulombs, C) that flowed through the cell.
We use the equation Q = It (that is, charge = current × time) to find that
Q = (5 amps)(1930 sec) = 9650 coulombs
Step 2: Use the faraday, F, to convert Q from Step 1 to moles of electrons.
The faraday is the magnitude of the charge on 1 mole of electrons; it’s a constant equal to (1.6 × 10−19 C/e−)(6.02 × 1023 e−/mol) ≈ 96,500 C/mol. So, if 9650 C of charge flowed through the cell, this represents
9650 C × 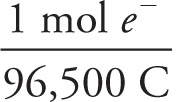 = 0.1 mol e−
= 0.1 mol e−
Step 3: Use the stoichiometry of the half-reactions to finish the calculation.
a) From the stoichiometry of the reaction Na+ + e− → Na, we see that 1 mole of electrons would give 1 mole of Na. Therefore, 0.1 mol of electrons gives 0.1 mol of Na. Since the molar mass of sodium is 23 g/mol, we’d get (0.1)(23 g) = 2.3 g of sodium metal deposited onto the cathode.
b) From the stoichiometry of the reaction 2 Cl− → Cl2(g) + 2e−, we see that for every 1 mole of electrons lost, we get ![]() mole of Cl2(g). Since Step 2 told us that 0.1 mol of electrons were liberated at the anode, 0.05 mol of Cl2(g) was produced. Because the molar mass of Cl2 is 2(35.5 g/mol) = 71 g/mol, we’d get (0.05 mol)(71 g/mol) = 3.55 g of chlorine gas.
mole of Cl2(g). Since Step 2 told us that 0.1 mol of electrons were liberated at the anode, 0.05 mol of Cl2(g) was produced. Because the molar mass of Cl2 is 2(35.5 g/mol) = 71 g/mol, we’d get (0.05 mol)(71 g/mol) = 3.55 g of chlorine gas.
Example 12-12: A piece of steel is the cathode in a hot solution of chromic acid (H2CrO4) to electroplate it with chromium metal. How much chromium would be deposited onto the steel after 48,250 C of electricity was forced through the cell?
A) ![]() mol
mol
B) ![]() mol
mol
C) ![]() mol
mol
D) 3 mol
Solution: First, we notice that 48,250 C of electricity is equal to ![]() faraday (F = 96,500 C/mol). This is equivalent to
faraday (F = 96,500 C/mol). This is equivalent to ![]() mole of electrons. In the molecule H2CrO4, chromium is in a +6 oxidation state. So, from the stoichiometry of the reaction Cr6+ + 6e− → Cr, we see that for every 6 moles of electrons gained, we get 1 mole of Cr metal. Another way of looking at this is to say that for every 1 mole of electrons gained, we get just
mole of electrons. In the molecule H2CrO4, chromium is in a +6 oxidation state. So, from the stoichiometry of the reaction Cr6+ + 6e− → Cr, we see that for every 6 moles of electrons gained, we get 1 mole of Cr metal. Another way of looking at this is to say that for every 1 mole of electrons gained, we get just ![]() mole of Cr metal. Therefore, if we have a supply of
mole of Cr metal. Therefore, if we have a supply of ![]() mol of electrons, we’ll produce (
mol of electrons, we’ll produce (![]() )(
)(![]() ) =
) = ![]() mol of Cr, choice A.
mol of Cr, choice A.
Chapter 12 Summary
• Oxidation is electron loss; reduction is electron gain (remember “OIL RIG”).
• A species that is oxidized is a reducing agent, and a species that is reduced is an oxidizing agent.
• In all electrochemical cells, oxidation occurs at the anode and reduction occurs at the cathode.
• Electrons always flow from the anode to the cathode.
• Salt bridge anions always migrate toward the anode, and cations always migrate toward the cathode.
• The free energy of an electrochemical cell can be calculated from its potential based on ∆G° = −nFE°.
• A galvanic cell spontaneously generates electrical power (−∆G, +E).
• An electrolytic cell consists of nonspontaneous reactions and requires an external electrical power source (+∆G, −E).
• In a galvanic cell, electrons spontaneously flow from the negative (−) terminal to the positive (+) terminal. Therefore, it follows that in a galvanic cell the anode is negatively charged (−) and the cathode is positively charged (+).
• In an electrolytic cell, electrons are forced from the positive (+) terminal to the negative (−) terminal, and therefore the anode is positively charged (+) and the cathode is negatively charged (−).
• Standard reduction and oxidation potentials are intrinsic values and therefore should not be multiplied by molar coefficients in balanced half reactions.
• For a given reduction potential, the reverse reaction, or oxidation potential, has the same magnitude of E but the opposite sign.
• Faraday’s law of electrolysis states that the amount of chemical change is proportional to the amount of electricity that flows through the cell.
• Under nonstandard conditions, the potential of an electrochemical cell can be calculated using the Nernst equation: E = E° − (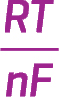 )lnQ
)lnQ
CHAPTER 12 FREESTANDING PRACTICE QUESTIONS
1. Typical dry cell batteries contain a zinc anode and a carbon cathode and produce a potential difference of 1.5 V. Given that many electronic devices require additional voltage, which of the following would result in an overall increase in voltage?
I. Doubling the quantity of Zn(s)
II. Placing two batteries in parallel
III. Replacing Zn(s) with Na(s)
A) I only
B) III only
C) I and II only
D) II and III only
2. Given the following reactions:
Pb2+ + 2e− → Pb(s) Eº = —0.13 V
Fe(s) → Fe2+ + 2e− Eº = 0.45 V
Which one of the following is true?
A) Pb(s) is a better reductant than Fe(s)
B) Fe(s) is a worse reductant than Pb(s)
C) Fe2+ is a better oxidant than Pb2+
D) Pb2+ is a better oxidant than Fe2+
3. Which of the following best characterizes the spontaneous half-reaction below under standard conditions?
Pd2+ + 2e− → Pd
A) ∆G° > 0 and E° < 0
B) ∆G° < 0 and E° < 0
C) ∆G° > 0 and E° > 0
D) ∆G° < 0 and E° > 0
4. High valent metals (those with large, positive oxidation states) are often used as strong oxidizing agents. Which of the following compounds would have the most positive reduction potential vs. a standard hydrogen electrode?
A) FeCl3
B) OsO4
C) Zn(NO3)Cl
D) W(CO)6
5. Which of the following best describes the difference between a galvanic cell and an electrolytic cell?
A) In a galvanic cell, the anode is the site of oxidation, whereas in an electrolytic cell the anode is the site of reduction.
B) In a galvanic cell, the cathode is the negative electrode, whereas in an electrolytic cell the cathode is the positive electrode.
C) In a galvanic cell, spontaneous reactions generate a current, whereas in an electrolytic cell a current forces nonspontaneous reactions to occur.
D) In a galvanic cell, the electrons flow from anode to cathode, whereas in an electrolytic cell the electrons flow from cathode to anode.
6. To give “white gold” a white appearance, it is plated with rhodium by immersion in a rhodium sulfate solution (Rh2(SO4)3(aq)). Provided with a current of 2.0 A, how long must a 3.0 g white gold broach be immersed to plate 3.0 × 10−5 g of rhodium? (Faraday’s constant = 96,500 C/mol e−)
A) 0.0009 s
B) 0.0098 s
C) 0.042 s
D) 0.56 s
7. A galvanic cell is constructed from two half-cells of platinum and iron. The half-reactions for these two elements are provided as follows:
Pt2+(aq) + 2e− → Pt (s) |
E° = +1.20 V |
Fe3+(aq) + 3e− → Fe (s) |
E° = —0.036 V |
Which of the following statements is true about the galvanic cell?
A) E° = 1.164 V, and Pt2+ is the reducing agent
B) E° = 1.164 V, and Fe3+ is the reducing agent.
C) E° = 1.236 V, and Pt2+ is the oxidizing agent.
D) E° = 1.236 V, and Fe3+ is the oxidizing agent.
8. An electrochemical cell is constructed using two inert electrodes in one chamber with an inert electrolyte. The binary compound ICl is dissolved in the electrolyte, current is applied, and I2 and Cl2 are produced. Which of the following statements is true?
A) Cl2 was produced by reduction at the cathode.
B) I2 was produced by oxidation at the cathode.
C) Cl2 was produced by oxidation at the cathode.
D) I2 was produced by reduction at the cathode.
CHAPTER 12 PRACTICE PASSAGE
A student follows the schematic below and sets up the following electrochemical cell at room temperature.
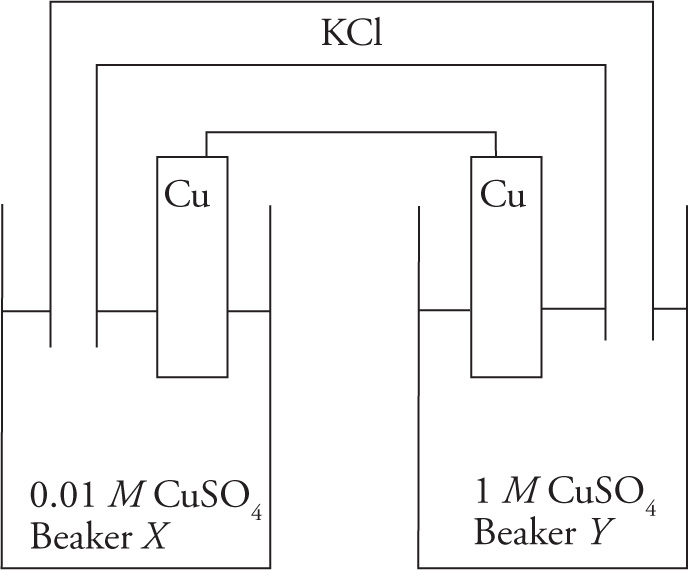
Figure 1 Schematic of an experimental electrochemical cell
The student fills Beaker X with a 0.01 M solution of CuSO4 and Beaker Y with a 1 M solution of CuSO4. Two copper plates serve as electrodes and a KCl(aq) salt bridge is used. Because of the nonstandard conditions, the student uses the Nernst equation to calculate the expected potential of the cell:
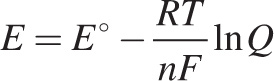
where E° is the standard cell voltage, R is the universal gas constant, T is the absolute temperature, n is the number of electron moles, F is Faraday’s constant, and Q is the reaction quotient. Substituting in the constant variables and converting the natural logarithm, the student obtains the following formula:
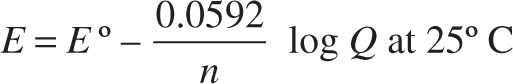
After setting up the half-cells, the student connects the two electrodes via a voltmeter and measures the initial cell potential.
Table 1 Standard reduction potentials for copper ions
Half-Reaction |
E° (V) |
Cu+ + e− → Cu(s) |
+ 0.52 |
Cu2+ + 2e− → Cu(s) |
+ 0.34 |
1. What is the standard cell voltage, E°, of the cell used in the experiment?
A) + 0.52 V
B) + 0.34 V
C) + 0.18 V
D) 0 V
2. Which of the following correctly describes the relationship between the movement of electrons and K+ ions?
A) K+ ions and electrons both travel towards Beaker X.
B) K+ ions travel towards Beaker X and electrons travel towards Beaker Y.
C) K+ ions travel towards Beaker Y and electrons travel towards Beaker X.
D) K+ ions and electrons both travel towards Beaker Y.
3. Which of the following substances has the atom with the greatest oxidation state?
A) CH2F2
B) K2O
C) S4O62—−
D) Fe2O3
4. Which of the following best describes the cell used in the experiment?
A) Beaker X contains the cathode that is negatively charged.
B) Beaker Y contains the cathode that is negatively charged.
C) Beaker X contains the anode that is negatively charged.
D) Beaker Y contains the anode that is negatively charged.
5. What is the expected potential of the cell at the start of the experiment?
A) +0.118 V
B) +0.059 V
C) −0.118 V
D) −0.059 V
SOLUTIONS TO CHAPTER 12 FREESTANDING PRACTICE QUESTIONS
1. B Increasing reagent quantity has no effect on voltage (Item I is incorrect, eliminating choices A and C), and placing batteries in parallel would leave the voltage unchanged (Item II is incorrect, eliminating choice D). Oxidation of zinc takes place at the anode, and sodium is a better reducing agent than zinc due to its lower ionization energy and tendency to give up an electron (Item III would result in an increase in voltage; the correct answer is choice B).
2. D Oxidant and reductant are synonymous with oxidizing agent and reducing agent, respectively. Choices A and B are saying the same thing, so both can be eliminated. To compare the relative strengths of the ions as oxidizing agents, reverse the half reaction for Fe so that it reads as a reduction:
Pb2+ + 2e− → Pb(s) |
E° = —0.13 V |
Fe2+ + 2e− → Fe(s) |
E° = —0.45 V |
Note that the sign of E° is reversed in this process. Pb2+ has a more positive reduction potential than Fe2+, making it the better oxidant.
3. D This question is asking about two factors (a two by two question): ∆G° and E°, which are related by ∆G° = −nFE°. For any spontaneous reaction, the change in Gibbs free energy (∆G°) is always less than 0 (eliminate choices A and C). As shown in the equation above, the standard reduction potential (E°) must be positive when ∆G° is negative, so eliminate choice B.
4. B A large, positive reduction potential indicates a strong tendency to be reduced, and hence ability to act as an oxidizing agent. The question states that high-valent metals act as strong oxidizing agents. Examining the oxidation states of the metals in question, we see that Fe = +3, Os = +8, Zn = +2, and W = 0. Therefore, since Os bears the largest positive oxidation state, we know that it is the strongest oxidizing agent.
5. C The anode is always the site of oxidation and the cathode is always the site of reduction; therefore, electrons always flow from the anode (oxidation) to the cathode (reduction) regardless of the kind of cell (choices A and D are wrong). In a galvanic cell, a spontaneous reaction liberates electrons and they flow freely to the positive electrode, which in this case would be the cathode. However, in an electrolytic cell the current is forcing the electrons to flow where they don’t want to go: the negative electrode. In this case the cathode would be the negative electrode (choice B is wrong).
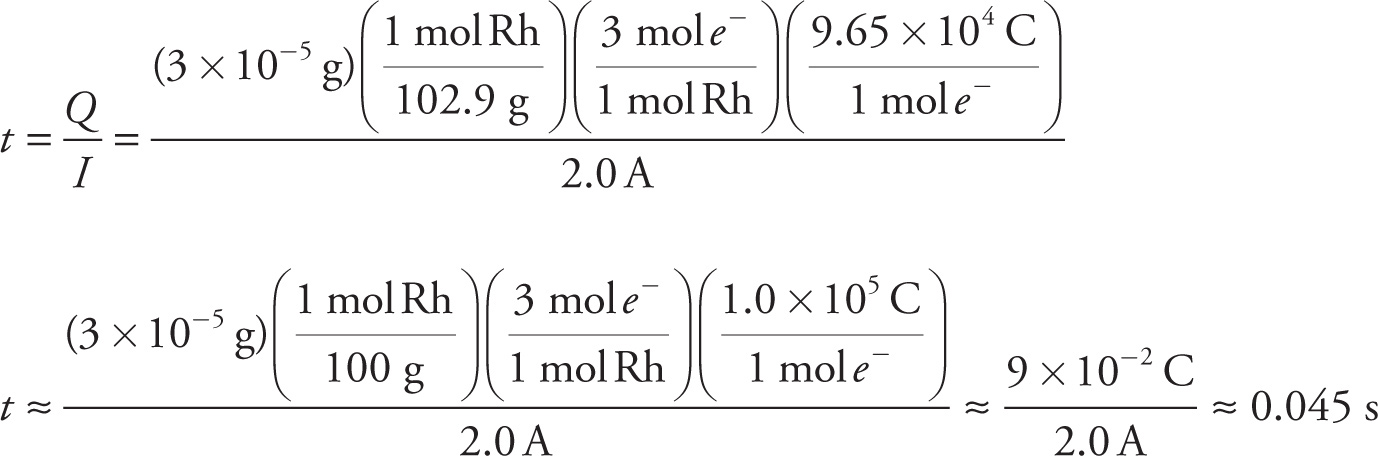
6. C Since current (I) = charge(Q) / time(t) we can set up and solve the following equation keeping in mind that the rhodium reduction in question is Rh3+ + 3e− → Rh(s).
7. C The half-reaction with the less positive reduction potential (in this case, Fe3+(aq) + 3e− → Fe(s)) should be reversed in order to combine the half-reactions to obtain an E° > 0 and create a galvanic cell. When the Fe half-reaction is reversed, the sign of the potential must be reversed. Combining the two half-reactions then gives: 3 Pt2+(aq) + 2 Fe(s) → 2 Fe3+(aq) + 3 Pt(s) with an E° = 1.236 V (eliminate choices A and B). Since Fe3+ is the product of the reaction, it cannot be the oxidizing agent (eliminate choice D). However, Pt2+ is reduced (it gains electrons from the Fe that is oxidized), so it is therefore the oxidizing agent.
8. D In the compound ICl, I has an +1 oxidation state, and Cl has a —1 oxidation state owing to the greater electronegativity of Cl. Therefore, production of Cl2 must be an oxidation, and production of I2 must be a reduction eliminating choices A and B. Moreover, reduction always takes place at the cathode, eliminating choice C.
SOLUTIONS TO CHAPTER 12 PRACTICE PASSAGE
1. D The setup shown is an example of a concentration cell. The difference in concentration will produce an electrical current, until the concentrations in both half-cells become equal. The standard cell voltage for this cell (and all concentration cells) is 0, because reciprocal redox reactions are occurring.
2. D No matter the type of cell, electrons always flow from anode to cathode. Also, oxidation always occurs at the anode and reduction always occurs at the cathode. In the concentration cell in the passage, [Cu2+] will increase in Beaker X and decrease in Beaker Y until they equalize. Therefore, oxidation occurs in Beaker X and reduction occurs in Beaker Y. Electrons flow from X to Y, and K+ ions follow the electrons to minimize the charge build-up in Beaker Y.
3. D Assigning oxidation states yields:
CH2F2: F = −1, H = 0, C = —2;
K2O: K = +1, O = −2;
S4O62−: O = −2, S = average of +2.5;
Fe2O3: O = −2, Fe = +3;
Since +3 is the greatest number, choice D is the best answer.
4. C Electrons always travel from anode to cathode. Loss of electrons, or oxidation, always occurs at the anode and gain of electrons, or reduction, always occurs at the cathode. A concentration cell is spontaneous, so electrons will spontaneously flow to a positive charge. Therefore, the cathode must be positively charged, eliminating choices A and B. Since [Cu2+] will increase in X and decrease in Y, X is the site of oxidation, or anode.
5. B A concentration cell is spontaneous, eliminating the negative values of potential in choices C and D. The net “reaction” of the concentration cell in the passage is:
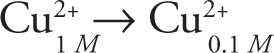
Therefore, the reaction quotient is:
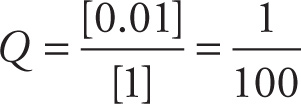
Copper is reacting between the Cu and Cu(II) state, so n = 2. Substitute these quantities into the Nernst equation to solve for potential: