MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
Acids and Bases
Equations to Remember
(10.1) Autoionization constant for water: Kw = [H3O+][OH−] = 10−14 at 25°C (298 K)
(10.2) Definitions of pH and pOH: 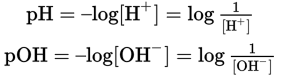
(10.3) Relationship of pH and pOH at 298 K: pH + pOH = 14
(10.4) p scale value approximation: p value ≈ m − 0.n
(10.5) Acid dissociation constant: ![]()
(10.6) Base dissociation constant: ![]()
(10.7) Relationship of Ka and Kb at 298 K: 
(10.8) Equivalence point: NaVa = NbVb
(10.9) Henderson—Hasselbalch equation (acid buffer): ![]()
(10.10) Henderson—Hasselbalch equation (base buffer): ![]()