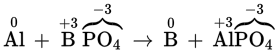MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
Oxidation–Reduction Reactions
Answers to Concept Checks
· 11.1
1.
|
Reaction |
Oxidation Numbers |
Oxidizing Agent |
Reducing Agent |
|
2 KI + H2 → 2 K + 2 HI |
|
K+ (charge goes from +1 to 0) |
H2 (charge goes from 0 to +1) |
|
Al + BPO4 → B + AlPO4 |
|
B3+ (charge goes from +3 to 0) |
Al (charge goes from 0 to +3) |
2. Oxidation: Zn → Zn2+ + 2 e—
Reduction: Cu2+ + 2 e— → Cu
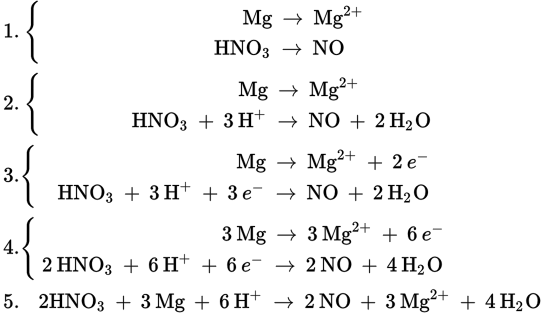
3. 
· 11.2
1. Cu+ + Cl— → CuCl
3 Mg + 2 Al3+ → 3 Mg2+ + 2 Al (don’t forget to balance the reaction!)
2. In the first reaction, chlorine undergoes disproportionation to have a —1 oxidation state in NaCl and a +5 oxidation state in NaClO3.
In the second reaction, sulfur undergoes disproportionation to have a 0 oxidation state in elemental sulfur and +4 oxidation state in SO2.
3. 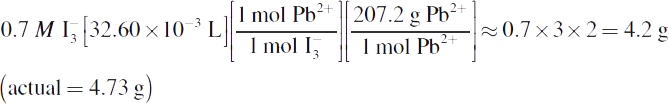
Note that question 3 also included the extraneous value 10.0 g, which is not needed to calculate the mass of lead produced.