MCAT General Chemistry Review - Alexander Stone Macnow, MD 2019-2020
The Gas Phase
Answers to Concept Checks
· 8.1
1. Gases are compressible fluids with rapid molecular motion, large intermolecular distances, and weak intermolecular forces.
2. At the top of the mountain, atmospheric pressure is lower, causing the column to fall. Under water, hydrostatic pressure is exerted on the barometer in addition to atmospheric pressure, causing the column to rise.
3. STP: T = 273 K (0°C), P = 1 atm
4. Standard conditions: T = 298 K (25°C), P = 1 atm, concentrations = 1 M
· 8.2
1. 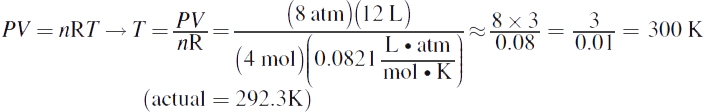
2. 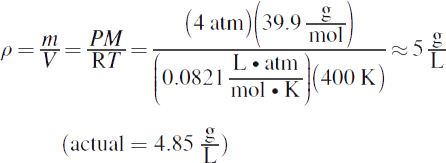
3. 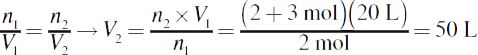
4. 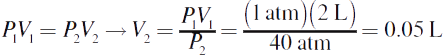
5. 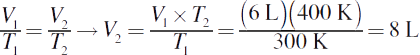
6. 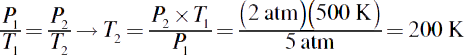
7. There are twelve total moles of gas, so the mole fractions of each gas are:
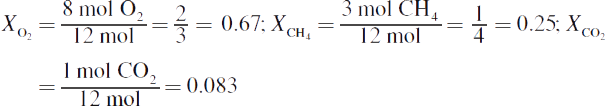
Then multiply each mole fraction by the total pressure to get the partial pressures (this is typically simpler with fractions than with decimals):
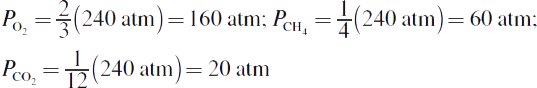
8. High pressures of carbon dioxide gas are forced on top of the liquid in sodas, increasing its concentration in the liquid.
· 8.3
1. Assumptions in the kinetic molecular theory include: negligible volume of gas particles, no intermolecular forces, random motion, elastic collisions, and proportionality between absolute temperature and energy.
2. 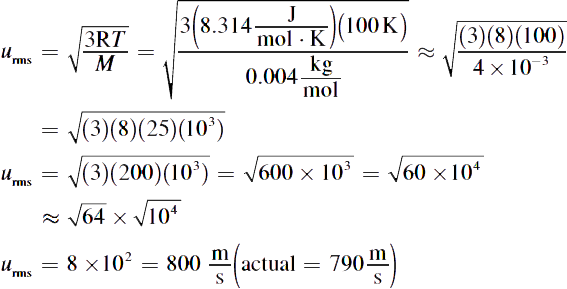
3. 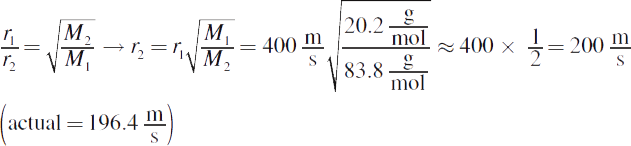
4. The rotten egg odor (hydrogen sulfide) first, almond (benzaldehyde) next, and wintergreen (methyl salicylate) last. Because all of the gases have the same temperature, they have the same kinetic energy; thus, the lightest molecules travel the fastest.
· 8.4
1. Real gas molecules have nonnegligible volume and attractive forces. Real gases deviate from ideal gases at high pressure (low volume) and low temperature.
2. According to the van der Waals equation, if a is increased while b remains negligible, the correction term ![]() gets larger, and the pressure drops to compensate. Therefore, methane will behave more ideally than chloromethane because a is smaller for methane. The real pressure of methane will thus be higher (closer to ideal).
gets larger, and the pressure drops to compensate. Therefore, methane will behave more ideally than chloromethane because a is smaller for methane. The real pressure of methane will thus be higher (closer to ideal).
3. Isobutane is larger and will thus have a larger correction term for the size of the molecule, b. This makes the term V - nb smaller. The pressure or volume must rise to compensate. Because the two gases are in the same size container, isobutane must exert a higher pressure.