March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 18. Rearrangements
18.B. Longer Nucleophilic Rearrangements
The question as to whether a group can migrate with its electron pair from A to C in W–A–B–C or over longer distances has been much debated. Although claims have been made that alkyl groups can migrate in this way, the evidence is that such migration is extremely rare, if it occurs at all. One experiment that demonstrated this was the generation of the 3,3-dimethyl-1-butyl cation (Me3CCH2CH2+). If 1,3-methyl migrations are possible, this cation would appear to be a favorable substrate, since such a migration would convert a primary cation into the tertiary 2-methyl-2-pentyl cation (Me2C+CH2CH2CH3), while the only possible 1,2 migration (of hydride) would give only a secondary cation. However, no products arising from the 2-methyl-2-pentyl cation were found, the only rearranged products being those formed by the 1,2-hydride migration.43 1,3-Migration of bromine has been reported.44
Most of the debate over the possibility of 1,3-migrations has concerned not methyl or bromine but 1,3-hydride shifts.45 Many instances have been found of apparent 1,3-hydride shifts, but the question is whether they are truly direct hydride shifts or whether they occur by another mechanism. There are at least two ways in
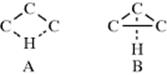
which indirect 1,3-hydride shifts can take place: (1) by successive 1,2-shifts or (2) through the intervention of protonated cyclopropanes (see Sec. 18.A.ii, category 2). A direct 1,3-shift would have transition state A, while the transition state for a 1,3-shift involving a protonated cyclopropane intermediate would resemble B. The evidence is that most reported 1,3-hydride shifts are actually the result of successive 1,2-migrations,46 but in some cases small amounts of products cannot be accounted for in this way. The reaction of 2-methyl-1-butanol with KOH and bromoform gave a mixture of alkenes, nearly all of which could have arisen from simple elimination or 1,2-shifts of hydride or alkyl. However, 1.2% of the product was 35.47 Hypothetically, 35 could have arisen from a 1,3 shift (direct or through a protonated cyclopropane) or from two successive 1,2-shifts:
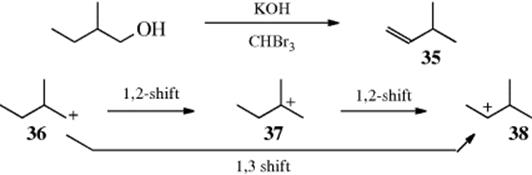
However, the same reaction applied to 2-methyl-2-butanol gave no 35, which demonstrated that 38 was not formed from 37. The conclusion made was that 38 was formed directly from 36. This experiment does not answer the question as to whether 38 was formed by a direct shift or through a protonated cyclopropane, but from other evidence48 it appears that 1,3-hydride shifts that do not result from successive 1,2-migrations usually take place through protonated cyclopropane intermediates, which (as seen in Sec. 18.A.ii, category 2) account for only a small percentage of the product in any case. However, there is evidence that direct 1,3-hydride shifts by way of A (see above) may take place in superacid solutions.49 Although direct nucleophilic rearrangements over distances >1,2 are rare (or perhaps nonexistent) when the migrating atom or group must move along a chain, this is not so for a shift across a ring of 8–11 members. Many such transannular rearrangements are known50 (see Sec. 4.Q.ii): the mechanism for the conversion of 39 to 40 is shown.51
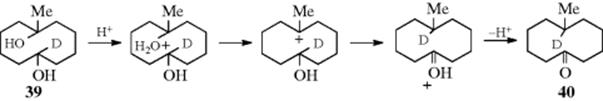
Note that the methyl group does not migrate in this system. It is generally true that alkyl groups do not undergo transannular migration.52 In most cases, it is the hydride that undergoes this type of migration, though a small amount of phenyl migration has also been shown.53