March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
4.D. Absolute Configuration
Suppose there are two test tubes, one containing (−)-lactic acid and the other the (+) enantiomer. One test tube contains 37 and the other 38. How can they be distinguished?
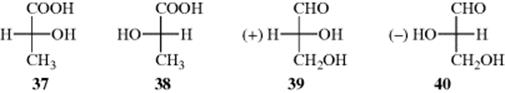
To generate a model to answer this question, Rosanoff proposed that one compound be chosen as a standard and a configuration arbitrarily assigned to it. The compound chosen was glyceraldehyde because of its relationship to the sugars. The (+) isomer was assigned the configuration shown in 39 and given the label D. The (−) isomer, designated to be 39, was given the label l. With a standard, other compounds could then be related to it. For example, (+)-glyceraldehyde, oxidized with mercuric oxide, gives (−)-glyceric acid:
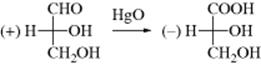
Since it is highly improbable that the configuration at the central carbon changed, it can be concluded that (−)-glyceric acid has the same configuration as (+)-glyceraldehyde and therefore (−)-glyceric acid is also called d. This example emphasizes that molecules with the same configuration need not rotate the plane of polarized light in the same direction. This fact should not surprise us when we remember that the same compound can rotate the plane in opposite directions under different conditions.
Once the configuration of the glyceric acids was known (in relation to the glyceraldehydes), it was then possible to relate other compounds to either of these, and each time a new compound was related, others could be related to it. In this way, many thousands of compounds were related, indirectly, to d- or l-glyceraldehyde. It was determined that 37, which has the d configuration, is the isomer that rotates the plane of polarized light to the left. Even compounds without asymmetric atoms (e.g., biphenyls and allenes), have been placed in the d or l series.98 When a compound has been placed in the d or l series, its absolute configuration is said to be known.99
In 1951, it became possible to determine that Rosanoff's guess was right. Ordinary X-ray crystallography cannot distinguish between a d and a l isomer, but by use of a special technique, Bijvoet et al.100 was able to examine sodium rubidium tartrate, compared it with glyceraldehyde, and found that Rosanoff had made the correct choice. It was perhaps historically fitting that the first true absolute configuration should have been determined on a salt of tartaric acid, since Pasteur made his great discoveries on another salt of this acid.
In spite of the former widespread use of d and l to denote absolute configuration, the method is not without faults. This method does not apply to all compounds that have a stereogenic center, but only those that can be structurally related to glyceraldehyde. The dl system is rarely used, therefore, except for certain groups of compounds (e.g., carbohydrates and amino acids). A more general model is required to distinguish the stereogenic centers of enantiomers.
4.D.i. The Cahn–Ingold–Prelog System
The system that is used universally is the Cahn–Ingold–Prelog system (or the CIP system), in which the four groups on a stereogenic carbon are ranked (prioritized) according to a set of sequence rules.101 For the most part, only a few of these rules are sufficient to deal with the vast majority of chiral compounds.
1. Prioritize substituents in order of decreasing atomic number of the atom directly joined to the carbon.
2. A tritium atom takes precedence over deuterium, which in turn takes precedence over ordinary hydrogen. Similarly, any higher isotope (e.g., 14C) takes precedence over any lower one.
3. Where two or more of the atoms connected to the stereogenic carbon are the same, the atomic number of the second atom determines the order. For example, in the molecule Me2CH–CHBr–CH2OH, the CH2OH group takes precedence over the Me2CH group because oxygen has a higher atomic number than carbon. Note that this is so even though there are two carbons in Me2CH, and only one oxygen in CH2OH. If two or more atoms connected to the second atom are the same, the third atom determines the precedence, and so on.
4. All atoms except hydrogen are formally given a valence of 4. Where the actual valence is less (as in nitrogen, oxygen, or a carbanion), phantom atoms (designated by a subscript 0) are used to bring the valence up to 4. These phantom atoms are assigned an atomic number of zero and necessarily rank lowest. Thus the ligand –+NHMe2 ranks higher than –NMe2.
5. Double and triple bonds are counted as if they were split into two or three single bonds, respectively, as in the examples in Table 4.1 (note the treatment of the phenyl group). Note that in a C=C double bond, the two carbon atoms are each regarded as being connected to two carbon atoms and that one of the latter is counted as having three phantom substituents.
Table 4.1 How Four Common Groups Are Treated in the Cahn–Ingold–Prelog System.
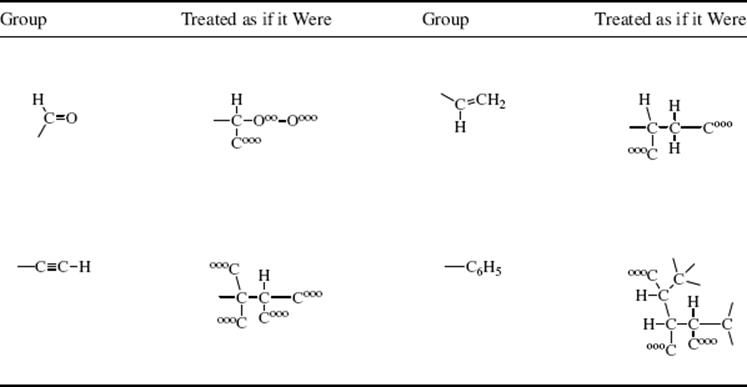
Using the four groups in Table 4.1 (aldehyde, vinyl, alkynyl, phenyl), the first atoms are connected, respectively, to (H, O, O), (H, C, C), (C, C, C), and (C, C, C). That is enough to establish that –CHO ranks first and –CH=CH2last, since even one oxygen outranks three carbons and three carbons outrank two carbons and a hydrogen. To classify the remaining two groups, proceed further along the chains. Note that –C6H5 has two of its (C, C, C) carbons connected to (C, C, H), while the third is (000) and is thus preferred to –C![]() CH, which has only one (C, C, H) and two (000)s.
CH, which has only one (C, C, H) and two (000)s.
By application of the above rules, some groups in descending order of precedence are COOH, COPh, COMe, CHO, CH(OH)2, o-tolyl, m-tolyl, p-tolyl, phenyl, C![]() CH, tert-butyl, cyclohexyl, vinyl, isopropyl, benzyl, neopentyl, allyl, n-pentyl, ethyl, methyl, deuterium, and hydrogen. Using the CIP rules, the four groups of glyceraldehyde are arranged in the sequence: OH, CHO, CH2OH, H.
CH, tert-butyl, cyclohexyl, vinyl, isopropyl, benzyl, neopentyl, allyl, n-pentyl, ethyl, methyl, deuterium, and hydrogen. Using the CIP rules, the four groups of glyceraldehyde are arranged in the sequence: OH, CHO, CH2OH, H.
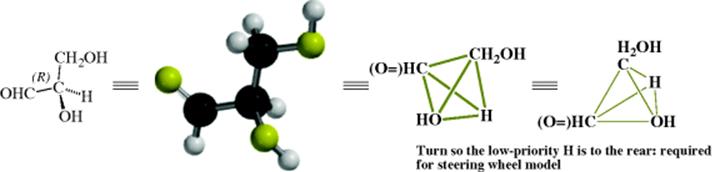
Once the order is determined, a model is required to determine the absolute configuration (i.e., which structure correlates to which enantiomer). The model used is known as the steering wheel model, where the molecule is held so that the lowest group in the sequence is pointed away from the viewer. Once the lowest priority group is held in that position, if the other groups, in the order listed, are oriented clockwise, the molecule is designated (R), and if counterclockwise (S). For glyceraldehyde, the (+) enantiomer is shown here and the CIP rules and the steering wheel model was used to assign an absolute configuration of (R).
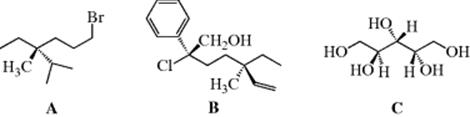
The CIP rules and steering wheel model are used to assign an absolute configuration to the following molecules. In A, the isopropyl carbon is higher in priority than the bromine-containing chain, and the methyl group is the lowest priority. Turning the molecule to place the methyl group to the rear makes this an (R) configuration. In B, there are two stereogenic centers, where the (S) center has the chain containing the (R) center as the lowest priority, and the (R) center has the methyl group as the low priority. In C, there are two (S) centers, but the hydroxyl-bearing carbon in the middle of the molecule is not a stereogenic carbon. Close inspection of C shows that this carbon has two identical groups [CH(OH)CH2OH].
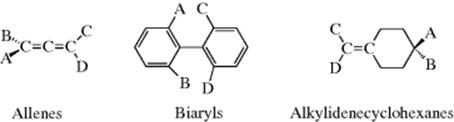
The CIP system is unambiguous and easily applicable in most cases. The CIP system also has been extended to chiral compounds that do not contain stereogenic centers, but rather have a chiral axis.102 Compounds having a chiral axis include unsymmetrical allenes, biaryls that exhibit atropisomerism (see Sec. 4.C, category 5), and alkylidene cyclohexane derivatives, molecular propellers and gears, helicenes, cyclophanes, annulenes, trans-cycloalkenes, and metallocenes. A series of rules have been proposed to address these cases based on what is called an “extended tetrahedron mode”, but the rules can be ambiguous in the case of cyclophanes and a few other systems.103
4.D.ii. Methods of Determining Configuration104
In all the methods,105 it is necessary to relate the compound of unknown configuration to another whose configuration is known. The most important methods of doing this follow:
1. Conversion of the Unknown to, or Formation of the Unknown from, a Compound of Known Configuration Without Disturbing the Stereogenic Center. The glyceraldehyde–glyceric acid example above is
![]()
one example. The stereogenic center was not disturbed, and the configuration of the product (glyceric acid) is the same as the starting material (glyceraldehyde). Retention of the same absolute configuration such as with glyceraldehyde-to-glyceric acid is not always the case. If the reaction sequence does not disturb (change) the stereogenic center, the absolute configuration depends on the nature of the groups. For example, when (R)-1-bromo-2-butanol is reduced to 2-butanol without disturbing the stereogenic center, the product is the (S) enantiomer because CH3CH2 ranks lower than BrCH2, but higher than CH3.
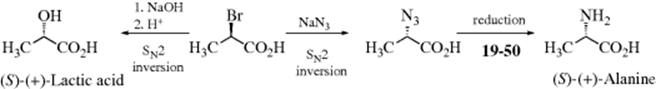
2. Conversion at the Stereogenic Center if the Mechanism is Known. An SN2 mechanism proceeds with inversion of configuration at a stereogenic carbon (Sec. 10.A.i). Indeed, a series of such transformations allowed the stereogenic center in lactic acid to be correlated to that in alanine.
3. Biochemical Methods. In a series of similar compounds (e.g., amino acids or certain types of steroids), a given enzyme will usually attack only molecules with one kind of configuration. If the enzyme attacks only the l form of eight amino acids, say, then attack on the unknown ninth amino acid will demonstrate that it is also the l form.
4. Optical Comparison. It is sometimes possible to use the sign and extent of rotation to determine which isomer has which configuration. In a homologous series, the rotation usually changes gradually and in one direction. If the configurations of enough members of the series are known, the configurations of the missing ones can be determined by extrapolation. Also, certain groups contribute more or less fixed amounts to the rotation of the parent molecule, especially when the parent is a rigid system (e.g., a steroid).
5. The Special X-Ray Method of Bijvoet. This method gives direct answers and has been used in a number of cases.86
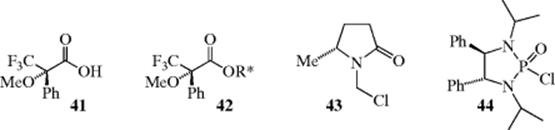
6. Derivatize the Alcohol with a Chiral Nonracemic Reagent and Examine the Ratio of Resulting Diastereomers by Gas Chromatography.106 This methods is one of the most useful for determining enantiomeric composition. Many derivatizing agents are available, but one class widely used are derivatives of α-methoxy-α-trifluoromethylphenyl acetic acid (MTPA, Mosher's acid, 41).107 Reaction with a chiral nonracemic alcohol (R∗OH) generates a Mosher's ester (42) that can be analyzed for diastereomeric composition by 1H or 19F NMR, as well as by chromatographic techniques.108 Complexation with lanthanide shift reagents allow the signals of the MTPA ester to be resolved and used to determine enantiomeric composition.109 This NMR method, as well as other related methods,110 are effective for determining the absolute configuration of an alcohol of interest (R∗OH, where R∗ is a group containing a stereogenic center).111 Two, of many other reagents that have been developed to determine the enantiopurity of alcohols and amines, include 43 and 44. Chloromethyl lactam (43) reacts with R∗OH or R∗NHR (R∗NH2),112 forming derivatives that allow analysis by 1H NMR and 44 reacts with alkoxides (R∗O−)113 to form a derivative that can be analyzed by 31P NMR. For a more detailed discussion of methods to determine optical purity, see Section 4.J.
7. Other Methods. Other method have also been used for determining absolute configuration in a variety of molecules, including optical rotatory dispersion,114 circular dichroism (CD),115 and asymmetric synthesis (Sec. 4.H). Optical rotatory dispersion (ORD) is a measurement of specific rotation [α] as a function of wavelength.116 The change of specific rotation [α] or molar rotation [Φ] with wavelength is measured, and a plot of either versus wavelength is often related to the sense of chirality or the substance under consideration. In general, the absolute value of the rotation increases as the wavelength decreases. The plot of CD is the differential absorption of left and right circularly polarized radiation by a nonracemic sample, taking place only in spectral regions in which absorption bands are found in the isotropic UV or visible (vis) electronic spectrum.117 The primary application of both ORD and CD is for the assignment of configuration or conformation.118 Configurational and conformational analyses have been carried out using IR and vibrational circular dichroism (VCD) spectroscopies.119
One example, and one of the more effective methods for derivatizing 1,2-diols, is the method employing dimolybdenum tetraacetate [Mo2(AcO)4] developed by Snatzke and Frelek.120 Exposure of the resulting complex to air leads, in most cases, to a significant induced CD spectrum (known as ICD). The method can be used for a variety of 1,2-diols.121
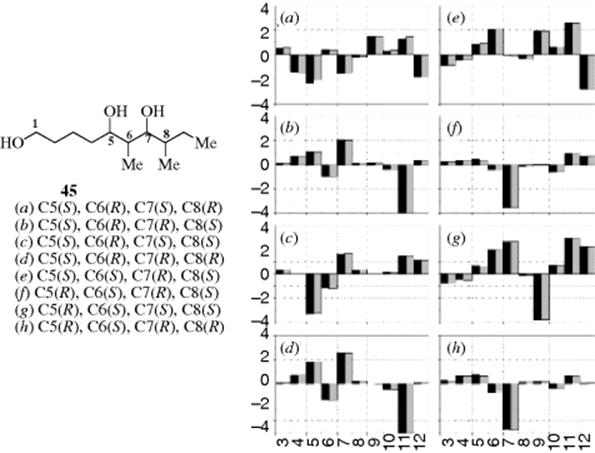
8. NMR Dabtabases. Kishi and co-worker's122 developed an NMR database123 of various molecules in chiral solvents, for the assignment of relative and absolute stereochemistry without derivatization or degradation. Kishi referred to this database as a “universal NMR database.”124 The diagram provided for diols 45 illustrates the method (see Fig. 4.3). The graph presents the difference in carbon chemical shifts between the average and the values for 45 (100 MHz) in DMBA (N,α-dimethylbenzylamine). Spectra were recorded in both enantiomers of the solvent, where the solid bar was recorded in (R)-DMBA and the shaded bar in (S)-DMBA. The x- and y-axes represent the carbon number and Δδ (δ45a–h - δave in ppm), respectively. The graphs are taken from “the 13C NMR database in (R)- and (S)-DMBA as a deviation in chemical shift for each carbon of a given diastereomer from the average chemical shift of the carbon in question. Each diastereomer exhibits an almost identical NMR profile for (R)- and (S)-DMBA, but shows an NMR profile distinct and differing from the other diastereomers, demonstrating that the database in (R)- and/or (S)-DMBA can be used for prediction of the relative stereochemistry of structural motifs in an intact form.”125
A 1H NMR analysis method has been developed that leads to the assignment of the stereochemistry of β-hydroxy ketones, by visual inspection of the ABX patterns for the (R)-methylene unit of the β-hydroxyketones.126Since β-hydroxy ketones are derived from the aldol reaction (16-34), this method is particularly useful in organic synthesis. A method has also been developed that uses 13C NMR to determine the relative stereochemistry of 2,3-dialkylpentenoic acids.127
Fig. 4.3 Proton NMR analysis for assignment of stereochemistry.