March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 6. Mechanisms and Methods of Determining Them
6.B. Types of Reaction
The number and range of organic reactions is so great as to seem bewildering, but actually almost all of them can be fitted into just six categories. In the description of the six types that follows, the immediate products are shown, although in many cases they then react with something else. All the species are shown without charges, since differently charged reactants can undergo analogous changes. The descriptions given here are purely for the purpose of classification and comparison. All are discussed in detail in Part II.
1. Substitutions. If heterolytic, these reactions can be classified as nucleophilic or electrophilic depending on which reactant is designated as the substrate and which as the attacking reagent (very often Y must first be formed by a previous bond cleavage).
a. Nucleophilic substitution (Chaps 10 and 13).
![]()
b. Electrophilic substitution (Chaps 11 and 12).
![]()
c. Free radical substitution (Chap 14).
![]()
In free radical substitution, Y√ is usually produced by a previous free radical cleavage, and X√ goes on to react further.
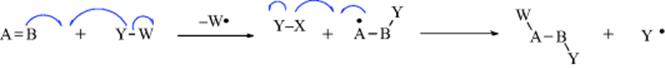
2. Additions to Double or Triple Bonds (Chaps 15 and 16). These reactions can take place by all three of the mechanistic possibilities.
a. Electrophilic addition (heterolytic).
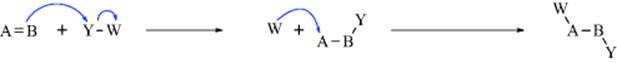
b. Nucleophilic addition (heterolytic).
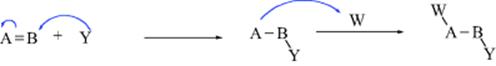
c. Free-radical addition (homolytic).
d. Simultaneous addition (pericyclic).

The examples show Y and W coming from the same molecule, but very often (except in simultaneous addition) they come from different molecules, as illustrated by (b). Cleavage of the Y–W bond may occur at the same time that Y is bonding to B, but the cleavage may also occur earlier.
3. β Elimination (Chap 17).

These reactions can take place by either heterolytic or pericyclic mechanisms. Examples of the latter are shown in Section 17.C.i. Free radical β eliminations are extremely rare. In heterolytic eliminations, W and X may or may not leave simultaneously and may or may not combine afterwards.
4. Rearrangement (Chap 18). Many rearrangements involve migration of an atom or group from one atom to another. There are three types, depending on how many electrons the migrating atom or group carries with it.
a. Migration with electron pair (nucleophilic; common).
![]()
b. Migration with one electron (free radical; rare).
![]()
c. Migration without electrons (electrophilic; rare).
![]()
The generic examples show 1,2 rearrangements, in which the migrating group moves to the adjacent atom. These are the most common, although longer rearrangements are also possible. There are also some rearrangements that do not involve simple migration at all, but rather migration across a π-framework (see Chap 18). Some of the latter involve pericyclic mechanisms.
5. Oxidation and Reduction (Chap 19). Many oxidation and reduction reactions fall naturally into one of the four types mentioned above, but many others do not. For a description of oxidation–reduction mechanistic types, see Section 19.A.
6. Combinations of the Above. Note that arrows are used to show movement of electrons. An arrow always follows the motion of electrons and never of a nucleus or anything else (it is understood that the rest of the molecule follows the electrons). Ordinary arrows (double-headed) follow electron pairs, while single-headed arrows follow unpaired electrons. Double-headed arrows are also used in pericyclic reactions for convenience, but in these reactions how or in which direction the electrons are moving is usually unknown.
While not mentioned here as a distinct category, it must be said that many reactions, including some examples of 1–6 are actually acid–base reactions. In other cases, an acid–base reaction initiates the process or sometimes ends the process. In 2a, for example, Y = H and this is an acid–base reaction in which the π bond is the base and the proton is the acid. If W = H in 3a, then the elimination process begins with an acid–base reaction in which a base donates two electrons to H (= W). In 2b, if A donates electrons to W and W = H, this is another example of an acid–base reaction. Always be mindful of the acid–base properties of reactions.
Many, if not most of the reactions, noted above are subject to modification of the reactivity by the introduction of π bonds. Most reactions involve the transfer of two electrons to make or break a bond. The presence of two electrons in a π bond allows this two- electron-transfer process to proceed through the intervening atoms. In effect, the reactivity of a given center is extended by the presence of π bonds. This is the concept of vinylogy: the extension of points of reactivity by intervening π bonds. In other words, if a system X—C-1—C-2 undergoes a reaction at C-2 with loss of X from C-1, X—C-1—C-2=C-3—C-4 may undergo reaction at C-4. Reaction at C-4 initiates electron transfer via the π bond that is extended to C-1 for loss of X. Several examples of this type of reaction will be presented in later chapters.