March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 6. Mechanisms and Methods of Determining Them
6.F. Kinetic and Thermodynamic Control
![]()
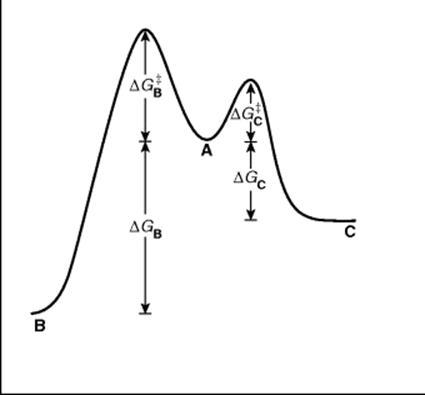
There are many cases in which a compound under a given set of reaction conditions can undergo competing reactions to give different products. Starting material A may give either B or C, for example. Figure 6.3 shows a free energy profile for a reaction in which B is thermodynamically more stable than C (ΔGB is > ΔGC), but C is formed faster (lower ΔG‡). If neither reaction is reversible, C will be formed in a larger amount because it is formed faster. The product is said to be kinetically controlled. However, if the reactions are reversible, this will not necessarily be the case. If such a process is stopped well before the equilibrium has been established, the reaction will be kinetically controlled since more of the faster-formed product will be present. However, if the reaction is permitted to approach equilibrium, the predominant or even exclusive product will be B. Under these conditions the C that is first formed reverts to A, while the more stable B does so much less. We say the product is thermodynamically controlled.23 Of course, Fig. 6.3 does not describe all reactions in which a compound A can give two different products. In many cases the more stable product is also the one that is formed faster. In such cases, the product of kinetic control is also the product of thermodynamic control.
Fig. 6.3 Free energy profile illustrating kinetic versus thermodynamic control of products. The starting compounds (A) can react to give either B or C.