March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 9. Effects of Structure and Medium on Reactivity
9.B. Steric Effects
It occasionally happens that a reaction proceeds much faster or much slower than expected on the basis of electrical effects alone. In these cases, it can often be shown that steric effects have a significant influence on the rate. For example, Table 9.2 lists relative rates for the SN2 ethanolysis of certain alkyl halides (see Sec. 10.A.i).3 All these compounds are primary bromides; the branching is on the second carbon, so that field-effect differences should be small. As Table 9.2 shows, the rate decreases with increasing b branching and reaches a very low value for neopentyl bromide. This reaction is known to involve an attack by the nucleophile from a position opposite to that of the bromine (see Sec. 10.A.i). The great decrease in rate can be attributed to steric hindrance in the transition state of the reaction, which makes attack of the nucleophile more difficult. Another example of steric hindrance is found in 2,6-disubstituted benzoic acids, which are difficult to esterify no matter what the resonance or field effects of the groups in the 2 or the 6 positions. Similarly, once 2,6-disubstituted benzoic acids are esterified, the esters are difficult to hydrolyze.
Table 9.2 Relative Rates of Reaction of RBr with Ethanola
R
Relative Rate
CH3
17.6
CH3CH2
1
CH3CH2CH2
0.28
(CH3)2CHCH2
0.030
(CH3)3CCH2
4.2 × 10−6
Reproduced from Hughes, E.D. Q. Rev. Chem. Soc. 1948, 2, 107 with permission from the Royal Society of Chemistry.
a. See Ref. 3.
Not all steric effects decrease reaction rates. In the hydrolysis of RCl by an SN1 mechanism (see Sec. 10.A.ii), the first step, which is rate determining, involves ionization of the alkyl chloride to a carbocation:

The central carbon in the alkyl chloride is sp3 hybridized, with angles of ~109.5°, but when it is converted to the carbocation, the hybridization becomes sp2 and the preferred angle is 120°. If the halide is tertiary and the three alkyl groups are large enough, they will be pushed together by the enforced tetrahedral angle, resulting in strain (see Sec. 4.Q.iv). This type of strain is called B strain4 (for back strain), and can be relieved by ionization to the carbocation.5
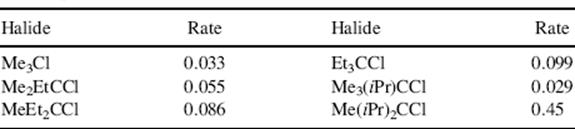
The rate of ionization (and hence the solvolysis rate) of a molecule in which there is B strain is expected to be larger than in cases where B strain is not present. Table 9.3 shows that this is so.6 Substitution of ethyl groups for the methyl groups of tert-butyl chloride does not cause B strain; the increase in rate is relatively small, and the rate smoothly rises with the increasing number of ethyl groups. The increase is caused by normal field and resonance (hyperconjugation) effects. Substitution by one isopropyl group is not greatly different. But with the second isopropyl group the crowding is now great enough to cause B strain, and the rate is increased 10-fold. Substitution of a third isopropyl group increases the rate still more. Another example where B strain increases the rate of solvolysis is found with the highly crowded molecules tri-tert-butylcarbinol, di-tert-butylneopentylcarbinol, tert-butyldineopentylcarbinol, and trineopentylcarbinol, where rates of solvolysis of the p-nitrobenzoate esters are faster than that of tert-butyl nitrobenzoate by factors of 13,000, 19,000, 68,000, and 560, respectively.7
Table 9.3 Rates of Hydrolysis of Tertiary Alkyl Chlorides at 25 °C in 80% Aqueous Ethanola
Reproduced with permission Brown, H.C.; Fletcher, R.S. J. Am. Chem. Soc. 1949, 71, 1845. Copyright © 1949 American Chemical Society.
a. See Ref. 6.
Another type of strain, which can affect rates of cyclic compounds, is called I strain (internal strain).8 This type of strain results from changes in ring strain in going from a tetrahedral to a trigonal carbon or vice versa. For example, as mentioned above, SN1 solvolysis of an alkyl halide involves a change in the bond angle of the central carbon from ~109.5° to ~120°. This change is highly favored in 1-chloro-1-methylcyclopentane because it relieves eclipsing strain (Sec. 4.Q.iv); thus this compound undergoes solvolysis in 80% ethanol at 25 °C, 43.7 times faster than the reference compound tert-butyl chloride.9 In the corresponding cyclohexyl compound, this factor is absent because the substrate does not have eclipsing strain (Sec. 4.Q.iv), and this compound undergoes the reaction at about one-third the rate of tert-butyl chloride. The reasons for this small decrease in rate are not clear. Corresponding behavior is found in the other direction, in changes from a trigonal to a tetrahedral carbon. Thus cyclohexanone undergoes addition reactions faster than cyclopentanone. Similar considerations apply to larger rings. Rings of 7–11 members exhibit eclipsing and transannular strain; and in these systems reactions in which a tetrahedral carbon becomes trigonal generally proceed faster than in open-chain systems.10 I-Strain has been shown to be a factor in other reactions as well.11

Conformational effects on reactivity can be considered under the heading of steric effects,12 but in these cases the effect of a group X and that of another group X′ upon reactivity at a site Y are not considered. But the effect of the conformation of the molecule must be considered. Many reactions fail entirely unless the molecules are able to assume the proper conformation. An example is the rearrangement of N-benzoylnorephedrine. The two diastereomers of this compound (4 and 5) behave very differently when treated with alcoholic HCl. In one of the isomers, nitrogen-to-oxygen migration takes place, while the other does not react at all.13 In order for the migration to take place, the nitrogen must be near the oxygen (gauche to it). When 4 assumes this conformation, the methyl and phenyl groups are anti to each other, which is a favorable position, but when 5 has the nitrogen gauche to the oxygen, the methyl must be gauche to the phenyl, which is so unfavorable that the reaction does not occur. Other examples are electrophilic additions to C=C double bonds (see Sec. 15.A.i) and E2 elimination reactions (see Sec. 17.A.i.). Also, many examples are known where axial and equatorial groups behave differently.14
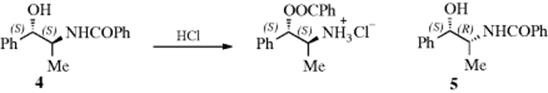
In steroids and other rigid systems, a functional group in one part of the molecule can strongly affect the rate of a reaction taking place at a remote part of the same molecule by altering the conformation of the whole skeleton. An example of this effect, called conformational transmission, is found in ergost-7-en-3-one (6) and cholest-6-en-3-one (7), where 7 condenses with benzaldehyde 15 times faster than 6.15 The reaction site in both cases is the carbonyl group, and the rate increases because moving the double bond from the 7 to the 6 position causes a change in conformation at the carbonyl group (the difference in the side chain at C-17 does not affect the rate). Molecular models of 6 and 7 are provided for illustration.