March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 1. Localized Chemical Bonding
1.F. Electronic Structures of Molecules
For each molecule, ion, or free radical that has only localized electrons, it is possible to draw an electronic formula, called a Lewis structure, which shows the location of these electrons. Only the valence electrons are shown. Valence electrons may be found in covalent bonds connecting two atoms or they may be unshared.23 Drawing these structures correctly is essential, since the position of electrons changes in the course of a reaction, and it is necessary to know where the electrons are initially before one can follow where they are going. To this end, the following rules operate:
1. The total number of valence electrons in the molecule (or ion or free radical) must be the sum of all outer-shell electrons “contributed” to the molecule by each atom plus the negative charge or minus the positive charge, for the case of ions. Thus, for H2SO4, there are 2 (one for each hydrogen) + 6 (for the sulfur) + 24 (6 for each oxygen) = 32; while for ![]() , the number is also 32, since each atom “contributes” 6 plus 2 for the negative charge.
, the number is also 32, since each atom “contributes” 6 plus 2 for the negative charge.
2. Once the number of valence electrons has been ascertained, it is necessary to determine which of them are found in covalent bonds and which are unshared. Unshared electrons (either a single electron or a pair) form part of the outer shell of just one atom, but electrons in a covalent bond are part of the outer shell of both atoms of the bond. First-row atoms (B, C, N, O, F) can have a maximum of eight valence electrons, and usually have this number, although some cases are known where a first-row atom has only six or seven. Where there is a choice between a structure that has six or seven electrons around a first-row atom and one in which all such atoms have an octet, the structure based on the octet is generally lower in energy than the one that is observed. For example, ethylene is
There are a few exceptions. For the molecule O2, the structure ![]() : has a lower energy than
: has a lower energy than ![]() :. Although first-row atoms are limited to 8 valence electrons, this is not so for second-row atoms, which can accommodate 10 or even 12 because empty d orbitals may be utilized.24 For example, PCl5 and SF6 are stable compounds, and the hybridization model can be used to explain this fact. In SF6, one s and one p electron from the ground state 3s23p4 of the sulfur are promoted to empty d orbitals, and the six orbitals hybridize to give six sp3d2 orbitals, which point to the corners of a regular octahedron.
:. Although first-row atoms are limited to 8 valence electrons, this is not so for second-row atoms, which can accommodate 10 or even 12 because empty d orbitals may be utilized.24 For example, PCl5 and SF6 are stable compounds, and the hybridization model can be used to explain this fact. In SF6, one s and one p electron from the ground state 3s23p4 of the sulfur are promoted to empty d orbitals, and the six orbitals hybridize to give six sp3d2 orbitals, which point to the corners of a regular octahedron.
3. It is customary to show the formal charge on each atom. For this purpose, an atom is considered to “own” all unshared electrons, but only one-half of the electrons in covalent bonds. The sum of electrons that thus “belong” to an atom is compared with the number “contributed” by the atom. An excess belonging to the atom results in a negative charge, and a deficiency results in a positive charge. The total of the formal charges on all atoms equals the charge on the whole molecule or ion. Note that the counting procedure is not the same for determining formal charge as for determining the number of valence electrons. For both purposes, an atom “owns” all unshared electrons, but for outer-shell purposes it “owns” both the electrons of the covalent bond, while for formal-charge purposes it “owns” only one-half of these electrons.
![]()
Examples of electronic structures are
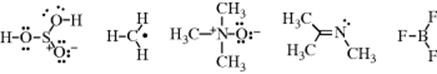
A coordinate-covalent bond (sometimes called a dative bond), represented by an arrow, is one in which both electrons come from the same atom; that is, the bond can be regarded as being formed by the overlap of an orbital containing two electrons with an empty one. Thus trimethylamine N-oxide would be represented:
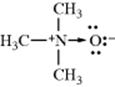
For a coordinate-covalent bond, the rule concerning formal charge is amended so that both electrons count for the donor and neither for the recipient. Thus the nitrogen and oxygen atoms of trimethylamine oxide bear no formal charges. However, it is apparent that the electronic picture is exactly the same as the picture of trimethylamine N-oxide given just above, and there is a choice of drawing an arrowhead or a charge separation. Some compounds (e.g., amine N-oxides) must be drawn one way or the other. It is usually simpler to use charge separation.