March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
II.C. Organic Syntheses References
At the end of many numbered sections there is a list of Organic Syntheses references (abbreviated OS). With the exception of a few very common reactions (12-3, 12–23, 12–24, and 12–38), and to the extent that it is possible, the list includes all OS references for each reaction. The volumes of OS that have been covered are Collective Volumes I–XI. There are indices to OS.6 Organic Syntheses can now be accessed online.7 Certain ground rules were followed in assembling these lists. A reaction in which two parts of a molecule independently undergo simultaneous reaction is listed under both reactions. Similarly, if two reactions happen (or might happen) rapidly in succession without the isolation of an intermediate, the reactions are listed in both places. For example, at OS IV, 266 is
![]()
This reaction is treated as Reaction 10–49 followed by Reaction 10–12 and is listed in both places. However, certain reactions are not listed because they are trivial examples. An instance of this is the reaction found at OS III, 468:
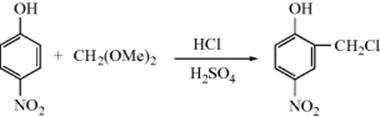
This is a chloromethylation reaction and is consequently listed in Reaction 11–14. However, in the course of the reaction formaldehyde is generated from the acetal. This reaction is not listed in Reaction 10-6 (hydrolysis of acetals), because it is not really a preparation of formaldehyde.
Notes
1. The classification of reactions into sections is, of course, to some degree arbitrary. Each individual reaction is different, and custom generally decides how we group them together. Individual preferences also play a part. No claim is made that the classification system used in this book is more valid than any other. For another way of classifying reactions, see Fujita, S. J. Chem. Soc., Perkin Trans. 2 1988, 597.
2. For a more complete set of rules, see Jones, R.A.Y.; Bunnett, J.F. Pure Appl. Chem. 1989, 61, 725.
3. For some examples, see: attachments (18-27, 19-29), detachments (19-72), simple rearrangements (18-7, 18-29), coupling (10-56, 19-34), uncoupling (19-9, 19-75), insertions (12-21, 18-9), extrusions (17-35, 17-38), ring opening (10-14, 10-35), ring closing (10-9, 15-60).
4. Guthrie, R.D. Pure Appl. Chem. 1989, 61, 23. For a briefer description, see Guthrie, R.D.; Jencks, W.P. Acc. Chem. Res. 1989, 22, 343.
5. There are actually two IUPAC systems. The one used in this book (Ref. 4) is intended for general use. A more detailed system, which describes every conceivable change happening in a system, and which is designed mostly for computer handling and storage, is given by Littler, J.S. Pure Appl. Chem. 1989, 61, 57. The two systems are compatible; the Littler system uses the same symbols as the Guthrie system, but has additional symbols.
6. Smith, M.B. Fieser and Fieser's Reagents For Organic Syntheses, Collective Index For Volumes 1–22, Wiley, New York, 2005; Smith, J.G.; Fieser, M. Fieser and Fieser's Reagents for Organic Synthesis: Collective Index for, Volumes 1–12, Wiley, New York, 1990; Liotta, D.C.; Volmer, M. Organic Syntheses Reaction Guide, Wiley: NY, 1991, which covers the series through Vol. 68. For an older index to Organic Syntheses (through Vol. 45), see Sugasawa, S.; Nakai, S. Reaction Index of Organic Syntheses, Wiley: NY, 1967.
7. Available at http://www.orgsyn.org/.