March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 10. Aliphatic Substitution, Nucleophilic and Organometallic
10.E. Nucleophilic Substitution at an Allylic Carbon: Allylic Rearrangements
Allylic substrates rapidly undergo nucleophilic substitution reactions (see Sec. 10.G.i, category 3), but we discuss them in a separate section because they are commonly accompanied by a certain kind of rearrangement known as an allylic rearrangement.226 When allylic substrates are treated with nucleophiles under SN1 conditions, two products are usually obtained: the normal one and a rearranged one.
![]()
Two products are formed because an allylic type of carbocation is a resonance hybrid
![]()
so that C-1 and C-3 each carry a partial positive charge and both are attacked by Y. Of course, an allylic rearrangement is undetectable in the case of symmetrical allylic cations, as in the case where R = H, unless isotopic labeling is used. This mechanism has been called the SN1′ mechanism. The IUPAC designation is 1/DN + 3/AN, the numbers 1 and 3 signifying the relative positions where the nucleophile attacks and from which the nucleofuge leaves.
As with other SN1 reactions, there is clear evidence that SN1′ reactions can involve ion pairs. If the intermediate attacked by the nucleophile is a completely free carbocation, then, say,
![]()
should give the same mixture of alcohols when reacting with hydroxide ion, since the carbocation from each should be the same. When treated with 0.8 M aq NaOH at 25 °C, 75 gave 60% CH3CH=CHCH2OH and 40% CH3CHOHCH=CH2, while 76 gave the products in yields of 38 and 62%, respectively.227 This phenomenon is called the product spread. In this case, and in most others, the product spread is in the direction of the starting compound. With increasing polarity of solvent,228 the product spread decreases and in some cases is entirely absent. It is evident that in such cases the high polarity of the solvent completely stabilizes free carbocations. There is other evidence for the intervention of ion pairs in many of these reactions. When H2C=CHCMe2Cl was treated with acetic acid, both acetates were obtained, but also some ClCH2CH=CMe3,229 and the isomerization was faster than the acetate formation. This could not have arisen from a completely free Cl− returning to the carbon, since the rate of formation of the rearranged chloride was unaffected by the addition of external Cl−. All these facts indicate that the first step in these reactions is the formation of an unsymmetrical intimate ion pair that undergoes a considerable amount of internal return and in which the counterion remains close to the carbon from which it departed. Thus, 75and 76, for example, give rise to two different intimate ion pairs. The field of the anion polarizes the allylic cation, making the nearby carbon atom more electrophilic, so that it has a greater chance of attracting the nucleophile.230
Nucleophilic substitution at an allylic carbon can also take place by an SN2 mechanism, in which case no allylic rearrangement usually takes place. However, allylic rearrangements can also take place under SN2 conditions, by the following mechanism, in which the nucleophile attacks at the γ carbon rather than the usual position:231
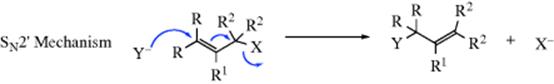
The IUPAC designation is 3/1/ANDN. This mechanism is a second-order allylic rearrangement; it usually comes about where SN2 conditions hold but where a substitution sterically retards the normal SN2 mechanism.232 There are few well-established cases of the SN2′ mechanism on substrates of the type C=C–CH2X, but compounds of the form C=C–CR2X give the SN2′ rearrangement almost exclusively233 when they give bimolecular reactions at all. Increasing the size of the nucleophile can also increase the extent of the SN2′ reaction at the expense of the SN2.233 In certain cases, the leaving group can also have an affect on whether the rearrangement occurs. Thus PhCH=CHCH2X, treated with LiAlH4, gave 100% SN2 reaction (no rearrangement) when X = Br or Cl, but 100% SN2′ when X = PPh3+ Br-.234 The solvent also plays a role in some cases, with more polar solvents giving more SN2′ product.235
The SN2′ mechanism, as shown above, involves the simultaneous movement of three pairs of electrons. However, Bordwell et al.236 contended that there is no evidence requiring that this bond making and bond breaking be in fact concerted, and that a true SN2′ mechanism is a myth. There is evidence both for237 and against238 this proposal. There is also a review of the SN′ reaction.239
The stereochemistry of SN2′ reactions has been investigated. It has been found that both syn240 (the nucleophile enters on the side from which the leaving group departs) and anti241 reactions can take place, depending on the nature of X and Y,242 although the syn pathway predominates in most cases.
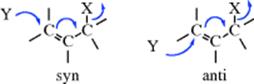
When a molecule has a nucleofuge capable of giving the SNi reaction in an allylic position, it is possible for the nucleophile to attack at the γ position instead of the α position. This reaction is called the SNi′ mechanism and has been demonstrated on 2-buten-1-ol and 3-buten-2-ol, both of which gave 100% allylic rearrangement when treated with thionyl chloride in ether.243 Ordinary allylic rearrangements (SN1′) or SN2′ mechanisms could not be expected to give 100% rearrangement in both cases. In the case shown, the nucleophile is only part of the leaving group, not the whole. But it is also possible to have reactions in which a simple leaving group, (e.g., Cl) comes off to form an ion pair244 and then returns not to the position where it ougmented but to the allylic position:

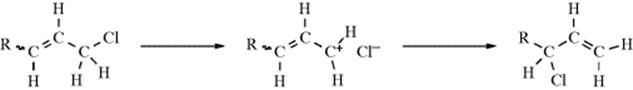
Most SNi′ reactions are of this type.
Allylic rearrangements have also been demonstrated in propargyl systems, for example,245
![]()
The product in this case is an allene,246 but such shifts can also give triple-bond compounds or, if Y = OH, an enol will be obtained that tautomerizes to an α,β-unsaturated aldehyde or ketone.
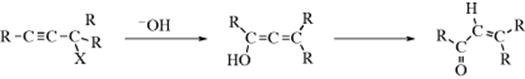
When X = OH, this conversion of acetylenic alcohols to unsaturated aldehydes or ketones is called the Meyer–Schuster rearrangement.247 The propargyl rearrangement can also go the other way; that is, 1-haloalkenes, treated with organocopper compounds, give alkynes.248
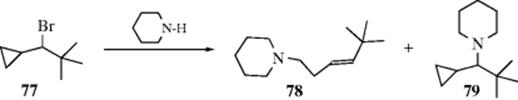
The SN2′ reaction has been shown to predominate in reactions of mixed cuprates (Reaction 10-58) with allylic mesylates,249 and in ring-opening reactions of aziridines.250 A related reaction is the opening of cyclopropylcarbinyl halides with organocuprates where the cyclopropane ring reacts similarly to the C=C unit of an alkene to give a homoallylic substituted product.251 This latter reaction is interesting since the reaction of 77 with piperidine leads to the SN2′ product (78) in ~ 87% yield, but there is ~ 8% of the direct substitution product, (79). Since the carbon bearing the bromine is very hindered, formation of 72 is somewhat unusual under these conditions. As Bordwell has suggested (see above), this may not be a true SN2 process.