Organic Chemistry I For Dummies, 2nd Edition (2014)
Part VI. Appendixes
Appendix C. Glossary
achiral:
A molecule that’s superimposable on its mirror image. Achiral molecules do not rotate plane-polarized light.
acid:
A proton donor or an electron pair acceptor.
alcohol:
A molecule containing a hydroxyl (OH) group. Also a functional group.
aldehyde:
A molecule containing a terminal carbonyl (CHO) group. Also a functional group.
alkane:
A molecule containing only C-H and C-C single bonds.
alkene:
A molecule containing one or more carbon-carbon double bonds. Also a functional group.
alkyne:
A molecule containing one or more carbon-carbon triple bonds. Also a functional group.
allylic carbon:
An sp3 carbon adjacent to a double bond.
amide:
A molecule containing a carbonyl group attached to a nitrogen (–CONR2). Also a functional group.
amine:
A molecule containing an isolated nitrogen (NR3). Also a functional group.
anion:
A negatively charged atom or molecule.
anti addition:
A reaction in which the two groups of a reagent X-Y add on opposite faces of a carbon-carbon bond.
anti conformation:
A type of staggered conformation in which the two big groups are opposite of each other in a Newman projection (see Figure C-1).
anti-aromatic:
A highly unstable planar ring system with 4n π electrons.
anti-periplanar (also known as anticoplanar):
The conformation in which a hydrogen and a leaving group are in the same plane and on opposite sides of a carbon-carbon single bond. The conformation required for E2 elimination.
aprotic solvents:
Solvents that do not contain O-H or N-H bonds.
aromatic:
A planar ring system that contains uninterrupted p orbitals around the ring and a total of 4n + 2 π electrons. Aromatic compounds are unusually stable compounds.
aryl:
An aromatic group as a substituent.
axial bond:
A bond perpendicular to the equator of the ring (up or down) in a chair cyclohexane (see Figure C-2).
base:
A proton acceptor or an electron pair donor.
benzyl group:
A benzene ring plus a methylene (CH2) unit (C6H5—CH2).
benzylic position:
The carbon attached to a benzene ring.
benzyne:
A highly reactive intermediate. A benzene ring with a triple bond (see Figure C-3).
bicyclic:
A molecule with two rings that share at least two carbons.
Brønsted acid:
A proton donor.
Brønsted base:
A proton acceptor.
carbanion:
A negatively charged carbon atom.
carbene:
A reactive intermediate, characterized by a neutral, electron-deficient carbon center with two substituents (R2C:).
carbocation:
A positively charged carbon.
carbonyl group:
A carbon double bonded to oxygen (C=O).
carboxylic acid:
A molecule containing a carboxyl (COOH) group. Also a functional group.
cation:
A positively charged molecule or atom.
chair conformation:
Typically, the most stable cyclohexane conformation. Looks like a chair (see Figure C-4).
chemical shift:
The location of an NMR peak relative to the standard tetramethylsilane (TMS), given in units of parts per million (ppm).
chiral center:
A carbon or other atom with four nonidentical substituents.
chiral molecule:
A molecule that’s not superimposable on its mirror image. Chiral molecules rotate plane-polarized light.
cis:
Two substituents on the same side of a double bond or ring.
configuration:
The three-dimensional orientation of atoms around a chiral center. It’s given the designation R or S.
conformation:
The instantaneous spatial arrangements of atoms. Conformations can change by rotation around single bonds.
conjugate acid:
The acid that results from protonation of a base.
conjugate base:
The base that results from the deprotonation of an acid.
conjugated double bonds:
Double bonds separated by one carbon-carbon single bond. Alternating double bonds.
constitutional isomers:
Molecules with the same molecular formula but with atoms attached in different ways.
coupling constant:
The distance between two neighboring lines in an NMR peak (given in units of Hz).
coupling protons:
Protons that interact with each other and split an NMR peak into a certain number of lines following the n + 1 rule.
covalent bond:
Bond in which the two electrons are shared between the two atoms.
dehydrohalogenation:
Loss of a hydrohalic acid (like HBr, HCl, and so on) to form a double bond.
delta value (also known as δ value):
The chemical shift. The location of an NMR peak relative to the standard tetramethylsilane (TMS), given in units of parts per million (ppm).
diastereomers:
Stereoisomers that are not mirror images of each other.
Diels–Alder reaction:
A reaction that brings together a diene and a dienophile to form cyclohexene rings.
diene:
A molecule that contains two alternating double bonds. A reactant in the Diels–Alder reaction.
dienophile:
A reactant in the Diels–Alder reaction that contains a double bond. Dienophiles are often substituted with electron-withdrawing groups.
dipole moment:
A measure of the separation of charge in a bond or molecule.
doublet:
An NMR signal split into two lines.
E isomer:
Stereoisomer in which the two highest-priority groups are on opposite sides of a double bond.
E1 elimination reaction:
A reaction that eliminates an acid (like HCl, HBr, and so on) to form an alkene. A first-order reaction that goes through a carbocation mechanism. It is the preferred mechanism for dehydration of secondary and tertiary alcohols to form alkenes.
E2 elimination reaction:
A reaction that eliminates an acid (like HCl, HBr, and so on) to form an alkene. A second-order reaction that occurs in single step, in which the double bond is formed as the hydrohalic acid is eliminated.
eclipsed conformation:
Conformation about a carbon-carbon single bond in which all the bonds of two adjacent carbons are aligned with each other (0 degrees apart when viewed in a Newman projection). (See Figure C-5.)
electronegativity:
The electron piggishness of an atom. More technically, a measure of the tendency of an atom to attract the electrons in a covalent bond to itself.
electrophile:
An electron lover. A molecule that can accept a lone pair of electrons (a Lewis acid).
enantiomers:
Molecules that are nonsuperimposable mirror images of each other.
equatorial:
The bonds in a chair cyclohexane that are oriented along the equator of the ring (see Figure C-6).
ester:
A molecule containing a carbonyl group adjacent to an oxygen bound to a carbon (RCOOR). Also a functional group.
ether:
A molecule containing oxygen singly bonded to two carbon atoms (R-O-R). Also a functional group. Often refers to diethyl ether.
fingerprint region:
Region of an IR spectrum below 1,500 cm–1. The fingerprint region of the IR spectrum is often complex and difficult to interpret.
functional group:
A reactivity center.
gauche conformation:
A type of staggered conformation in which two big groups are next to each other (shown in a Newman projection in Figure C-7).
halide:
A member of the VIIA column of the Periodic Table (like F, Cl, Br, I, and so on) or a molecule that contains one of these atoms. Also a functional group.
Hückel’s rule:
A rule that states that completely conjugated planar rings with 4n + 2π electrons are aromatic.
hybrid orbitals:
Orbitals formed from mixing together atomic orbitals, like the spx orbitals, which result from mixing s and p orbitals.
hyperconjugation:
Weak interaction (electron donation) between sigma bonds with p orbitals. Hyperconjugation helps explain why alkyl substituents stabilize carbocations.
inductive effects:
Electron donation or withdrawal by electropositive or electronegative atoms through the sigma bond framework. Inductive effects help explain why alkyl substituents stabilize carbocations.
intermediate:
Any species formed in a reaction on the way to making the product. Typically, intermediates are unstable.
ionic bond:
A bond in which the electrons are unshared between two atoms.
IR spectroscopy:
An instrumental technique that measures IR light absorption by molecules. Can be used to determine functional groups in an unknown molecule.
isolated double bonds:
Double bonds separated by more than one carbon-carbon single bond.
J value:
The coupling constant between two peaks in an NMR signal. Given in units of Hz.
ketone:
A compound that contains a carbonyl group attached to two carbons (RCOR). Also a functional group.
kinetic product:
The product that forms the fastest. (This product has the lowest energy of activation.)
kinetics:
The study of reaction rates.
Lewis acid:
An electron-pair acceptor.
Lewis base:
An electron-pair donor.
Markovnikov’s rule:
A rule that states that electrophiles add to the less highly substituted carbon of a carbon-carbon double bond (or the carbon with the most hydrogen atoms).
mass spectrometry:
An instrumental technique involving the ionization of molecules into fragments. Can be used to determine the molecular weights of unknown molecules.
meso:
Molecules that have chiral centers but are achiral as a result of one or more planes of symmetry in the molecule.
meta:
The positions of two substituents on a benzene ring that are separated by one carbon (see Figure C-8).
meta-directing substituent:
Any substituent on an aromatic ring that directs incoming electrophiles to the meta position.
molecular ion:
The fragment in a mass spectrum that corresponds to the cation radical (M·+) of the molecule. The molecular ion gives the molecular mass of the molecule.
molecular orbital theory:
A model for depicting the location of electrons that allows electrons to delocalize across the entire molecule. A more accurate but less user-friendly theory than the valence-bond model.
multistep synthesis:
Synthesis of a compound that takes several steps to achieve.
n + 1 rule:
A rule for predicting the coupling for a proton in 1H NMR spectroscopy. An NMR signal will split into n + 1 peaks, where n is the number of equivalent adjacent protons.
natural product:
A compound produced by a living organism.
nitrile:
A compound containing a cyano group, a carbon triply bonded to a nitrogen (CN). Also a functional group.
NMR:
Nuclear magnetic resonance sprectroscopy. A technique that measures radio frequency light absorption by molecules. A powerful structure-determining method.
node:
A region in an orbital with zero electron density.
nucleophile:
A nucleus lover. A molecule with the ability to donate a lone pair of electrons (a Lewis base).
nucleophilicity:
A measure of the reactivity of a nucleophile in a nucleophilic substitution reaction.
optically active:
Rotates plane-polarized light.
orbital:
The region of space in which an electron is confined (the electron “apartment”).
organic compound:
A carbon-containing compound.
ortho:
The positions of two substituents on a benzene ring that are on adjacent carbons (see Figure C-9).
ortho-para director:
An aromatic substituent that directs incoming electrophiles to the ortho or para positions.
oxidation:
A reaction that involves the loss of electrons by an atom or molecule.
para:
Describes the positions of two substituents on a benzene ring that are separated by two carbons (see Figure C-10).
phenyl ring:
A benzene ring as a substituent, sometimes abbreviated Ph.
pi bond (also known as π bond):
A bond with electron density above and below the two atoms, but not directly between the two atoms. Found in double and triple bonds.
pKa:
The scale for defining a molecule’s acidity (pKa = –log Ka).
plane of symmetry:
A plane cutting through a molecule in which both halves are mirror images of each other.
plane-polarized light:
Light that oscillates in a single plane.
protic solvent:
A solvent that contains O-H or N-H bonds.
proton:
An H+ ion. Also a positively charged nuclear particle.
R group:
Abbreviation given to an unimportant part of a molecule. Indicates the rest of the molecule.
Racemic mixture:
A 50/50 mixture of two enantiomers. Racemic mixtures are optically inactive (they don’t rotate plane-polarized light).
radical:
An atom or molecule with an unpaired electron.
reduction:
A reaction involving the gain of electrons by an atom or molecule.
resonance structures:
Structures used to better depict the location of pi and nonbonding electrons on a molecule. A molecule looks like a hybrid of all resonance structures.
s-cis conformation:
A conformation in which the two double bonds of a conjugated diene are on the same side of the carbon-carbon single bond that connects them (see Figure C-11). The required conformation for the Diels–Alder reaction.
s-trans conformation:
The conformation in which the two double bonds of a conjugated diene are on opposite sides of the carbon-carbon single bond that connects them (see Figure C-12).
sigma bond (also known as σ bond):
A bond in which electrons are located between the nuclei of the bonding atoms. Single bonds are sigma bonds.
singlet:
Describes an NMR signal consisting of only one line.
SN1 reaction:
A first-order substitution reaction that goes through a carbocation intermediate.
SN2 reaction:
A second-order substitution reaction that takes place in one step and has no intermediates.
sp
A hybrid orbital made by mixing one s orbital and one p orbital. The angle between sp orbitals is usually about 180 degrees.
sp2
A hybrid orbital made by mixing one s orbital and two p orbitals. The angle between sp2 orbitals is usually about 120 degrees.
sp3:
A hybrid orbital made by mixing one s orbital and three p orbitals. The angle between sp3 orbitals is usually about 109.5 degrees.
staggered conformation:
Conformation about a carbon-carbon single bond in which bonds of one carbon are at a maximum distance apart from bonds coming off of an adjacent carbon (60 degrees apart when viewed in a Newman projection). (See Figure C-13.)
stereochemistry:
The study of molecules in three dimensions.
stereoisomers:
Molecules that have the same atom connectivity, but different orientations of those atoms in three-dimensional space.
steric hindrance:
The way that atoms can shield a site by getting in the way of approach of a reactant.
substituent:
A piece that sticks off the main carbon chain or ring.
syn addition:
A reaction in which two groups of a reagent X-Y add on the same face of a carbon-carbon double bond.
tautomers:
Molecules that differ in the placement of a hydrogen and double bonds and are easily interconvertible. Keto and enol forms are tautomers.
thermodynamic product:
The reaction product with the lowest energy.
thermodynamics:
The study of the energies of molecules.
thiol:
A molecule containing an SH group. Also, a functional group.
transition state:
The structure that corresponds to the highest point on the energy hill that takes one species into another.
triplet:
An NMR signal split into three lines.
Z isomer:
An isomer in which the two highest-priority substituents are on the same side of a double bond.
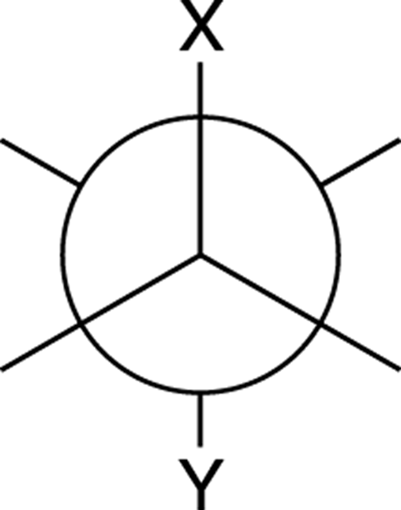
FIGURE C-1: A Newman projection of an anti conformation.
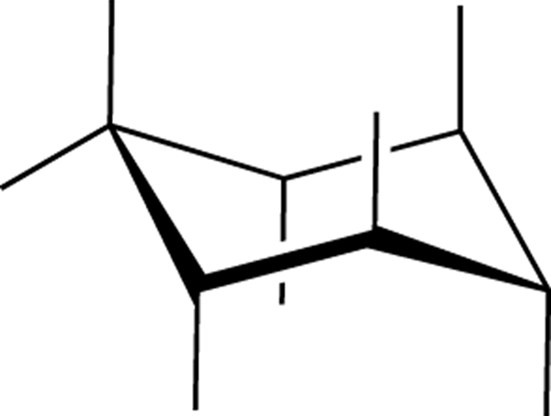
FIGURE C-2: Axial bonds.
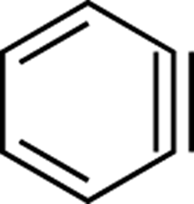
FIGURE C-3: Benzyne.
FIGURE C-4: Chair conformation.

FIGURE C-5: A Newman projection of eclipsed conformation.
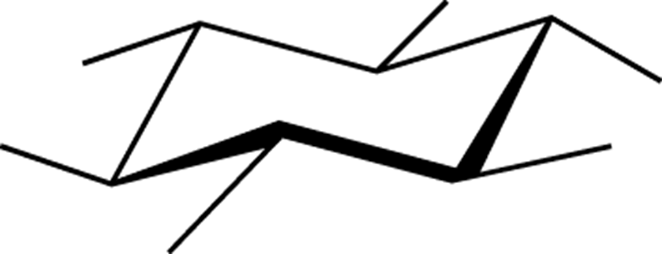
FIGURE C-6: Equatorial.

FIGURE C-7: A Newman projection of a gauche conformation.

FIGURE C-8: Meta.
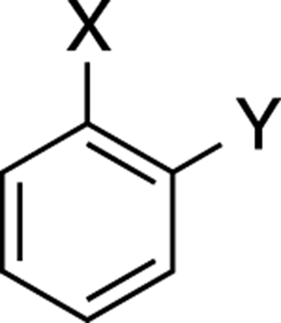
FIGURE C-9: Ortho.
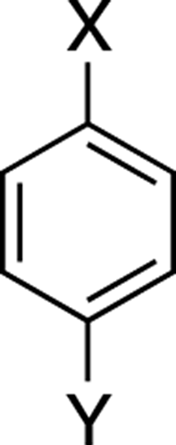
FIGURE C-10: Para.

FIGURE C-11: s-cis conformation.
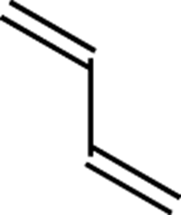
FIGURE C-12: s-trans conformation.

FIGURE C-13: A Newman projection of a staggered conformation.