Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Oxidation Reactions in Organic Chemistry
11.6 Applications of Oxidation Reactions of Alkenes
In this section, we apply our knowledge of oxidation reactions to determine the structures of unknown starting compound by thinking in reverse, this process requires analytical thinking! The first type of reaction that we will examine is the use of KMNO4 for the oxidation of alkenes. Reaction (11-75) shows the oxidation of an unknown starting compound, and it was demonstrated from a separate experiment that the unknown compound discolored bromine in the presence of carbon tetrachloride as solvent.
(11-75)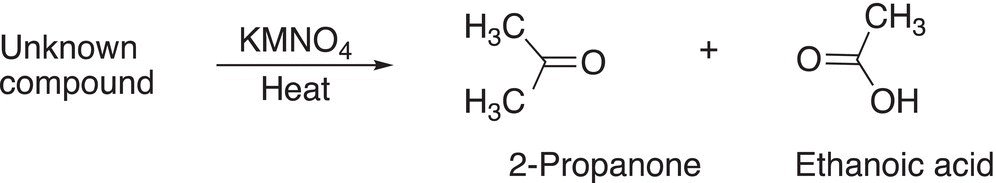
In order to determine the structure of the unknown starting reactant, we will have to make some very important observations before a conclusion can be made about the structure of this unknown compound. The first is that that it discolors bromine solution. You will recall from Chapter 8 that the reddish color of a solution of bromine solution becomes colorless when added to alkenes due to the addition reaction that occurs. Thus, this information implies that the unknown compound is an alkene. The next observation is that the reagent that is used for reaction in Reaction (11-75) is a strong oxidizing reagent in the presence of heat, which would cleave a carbon—carbon double bond to produce the products shown. Based on the structures of the products, the conclusion is that the unknown compound has the structure shown in Reaction (11-76), which also shows the carbon—carbon double bond that must be cleaved to produce the products shown.
(11-76)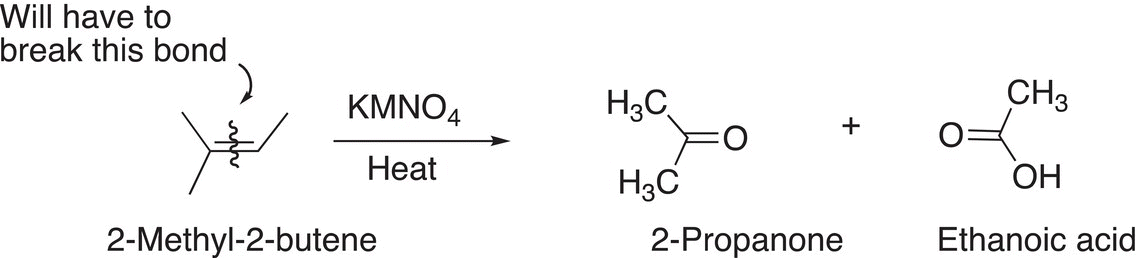
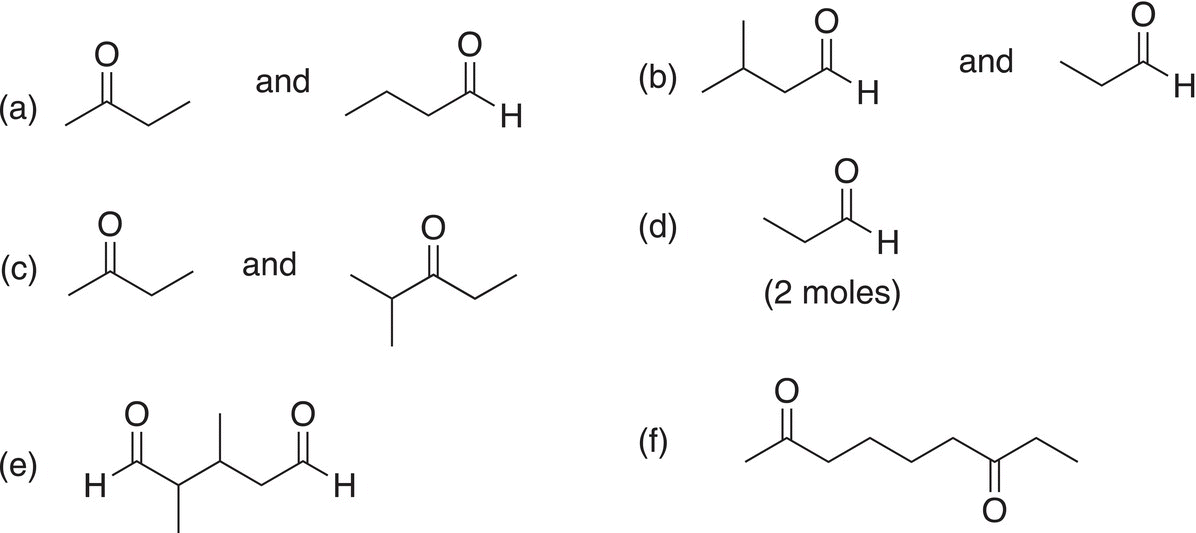
Problem 11.14
For each of the following pairs of compounds, determine the reactants based on the reaction with KMnO4 at elevated temperatures.
Another example of the application of analytical thinking to determine the structure of the reactant of an oxidation reaction is shown in Reaction (11-77). For this reaction, an unknown compound gave only one product and it is difunctional and was shown from a separate experiment that the unknown compound is an alkene.
(11-77)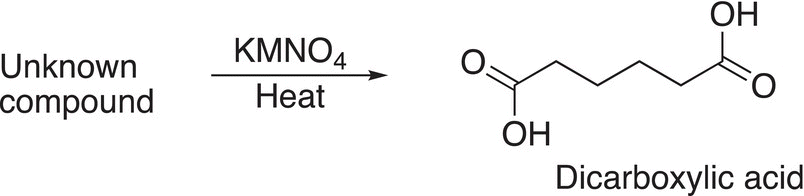
Utilizing this information, we can conclude that since the only product obtained is a difunctional carboxylic acid, the unknown reactant is more than likely cyclic and is oxidized to the dialdehyde and further oxidized to the dicarboxylic acid as shown in Reaction (11-78).
(11-78)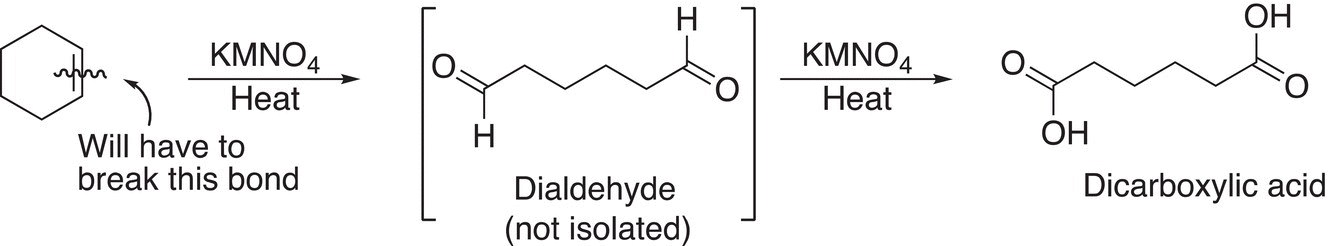
Again, since there is only one product obtained, it is obvious that the reactant is a cyclic alkene and that it has six carbons. A good conclusion is that it has to be cyclohexene.
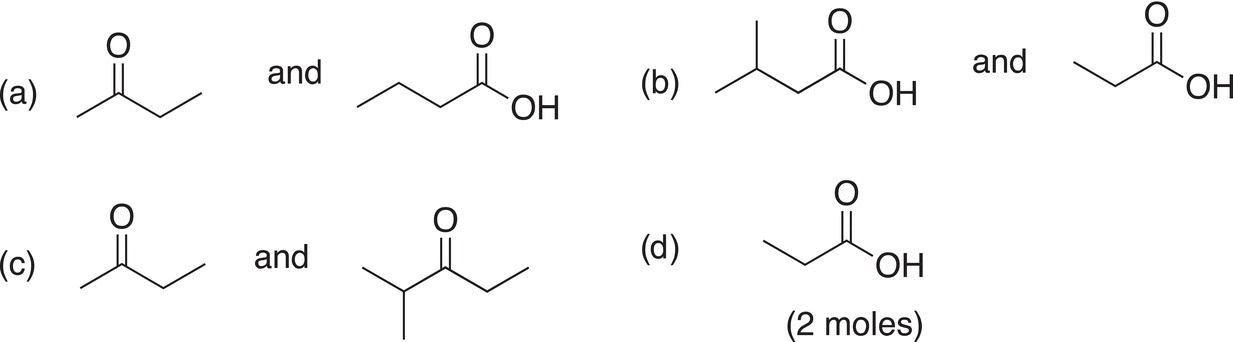
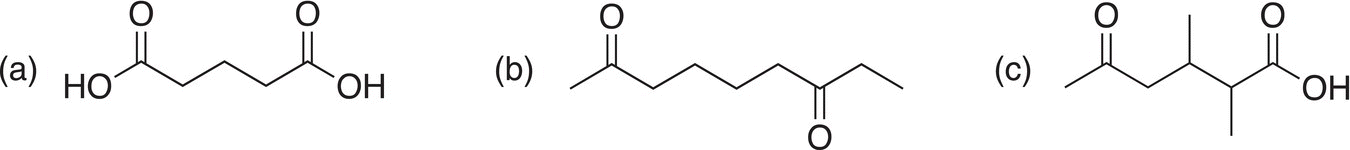
Problem 11.15
The following difunctional molecules were obtained from separate reactions carried out in KMnO4 at elevated temperatures. Determine the structure of the starting alkenes.
The same analysis can be carried out for ozonolysis to determine the structure of unknown compounds, but for these reactions, aldehydes can be isolated, as shown for the reactions shown in Reaction (11-79).
(11-79)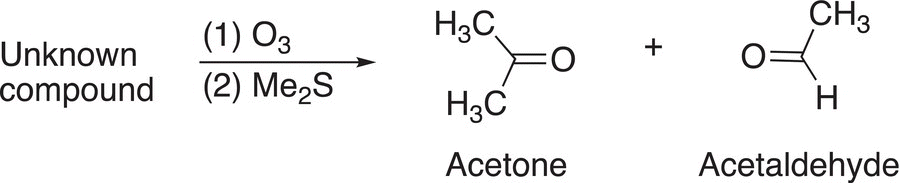
Using a similar approach as outlined for the use of KMnO4, the conclusion of the unknown reactant is shown in the reaction given in Reaction (11-80).
(11-80)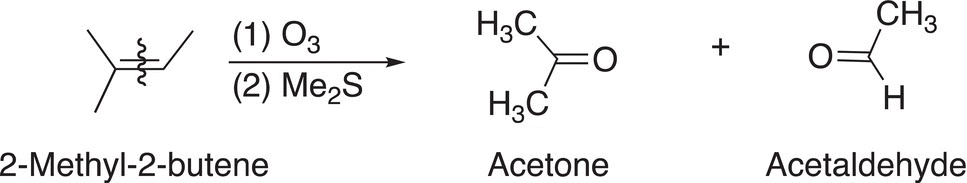
Another example is shown in Reaction (11-81), but in this case only one production is obtained and it is a dialdehyde.
(11-81)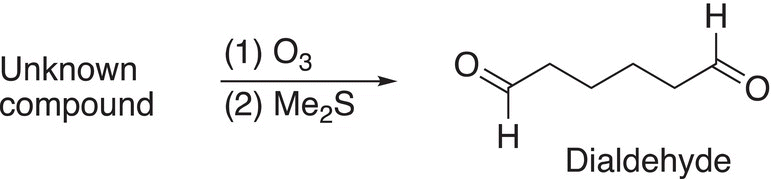
Since there is only one product obtained, it is obvious that the reactant is a cyclic alkene and that it has six carbons. A good conclusion is that the unknown reactant is cyclohexene, as shown in Reaction (11-82).
(11-82)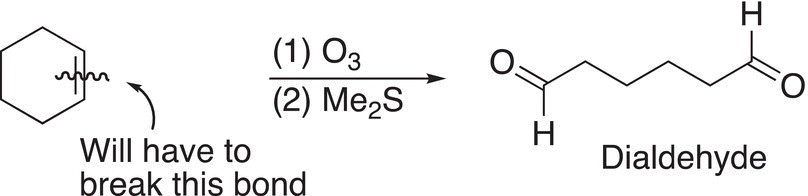
Problem 11.16
The following products were obtained from separate ozonolysis reactions of unknown compounds. Determine the structure of the starting alkene for each unknown.