Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Elimination Reactions of Organic Chemistry
12.2 Mechanisms of Elimination Reactions
One of the mechanisms that will be examined in this chapter involves the proton and the leaving group departing at the same time, also called a concerted reaction. For a reaction to proceed through this type of mechanism, typically a base is introduced in the reaction mixture to assist in the abstraction of hydrogen as a proton (i.e. hydrogen without its bonding electrons) to form the carbon—carbon double bond. This mechanism is referred to as bimolecular elimination (E2). Another mechanism by which the reaction can take place involves two steps. In the first step, the leaving group departs taking its bonding electrons, and in a different step, the proton is abstracted and its bonding electrons form the carbon—carbon double bond. This mechanism is referred to as unimolecular elimination (E1). Another possibility that will not be examined in this chapter involves the proton being abstracted first to form a conjugate base, then the electrons of this intermediate fold in to form a double bond while the leaving group leaves. This mechanism is called a unimolecular elimination, conjugate base (E1cB) mechanism. The first two mechanisms will be discussed in details in the next sections of this chapter.
12.2.1 Elimination Bimolecular (E2) Reaction Mechanism
If the reaction takes place in a manner so that the proton (H+) and leaving group leave at the same time to form the carbon—carbon double bond in a single step, the mechanism is described as bimolecular (E2). This type of mechanism is described as bimolecular because two molecules are involved in this very important step of the reaction. A base accomplishes the removal of the proton at the same time as the departure of the leaving group with its bonding pair of electrons; the electrons from the C─H bond forms the double bond in the product, as shown in Figure 12.1.
Since this reaction occurs in a single step, there is only one transition state and no intermediates for this type of reaction. The dotted lines shown in the transition state represent partial bonds that are being formed and broken as the reaction proceeds from reactants to products. The energy profile for this type of single-step reaction is shown in Figure 12.2. In this mechanism, the reactants proceed directly to the products through the transition state and then to products.
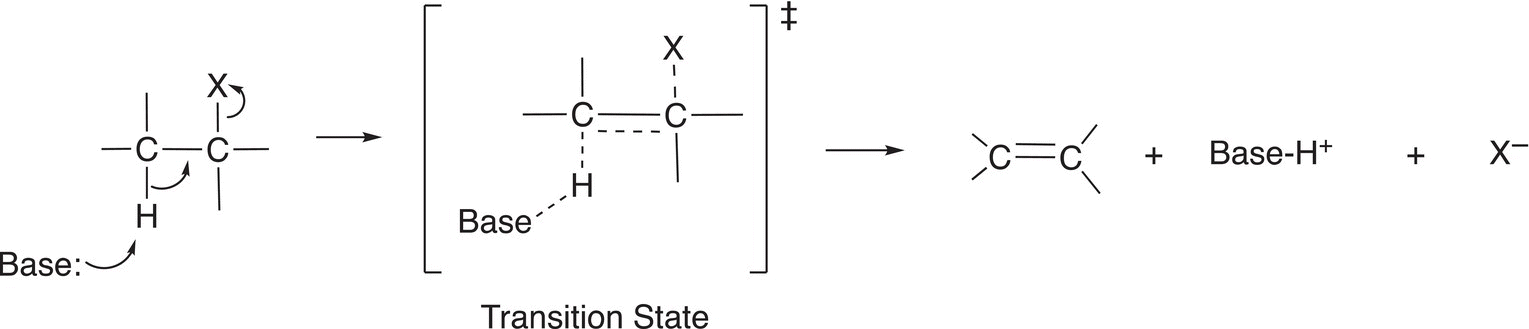
Figure 12.1 Mechanism for a β-elimination-bimolecular (E2) elimination reaction in which as Lewis base is used to abstract a proton from the β-carbon adjacent to the carbon that has the leaving group.
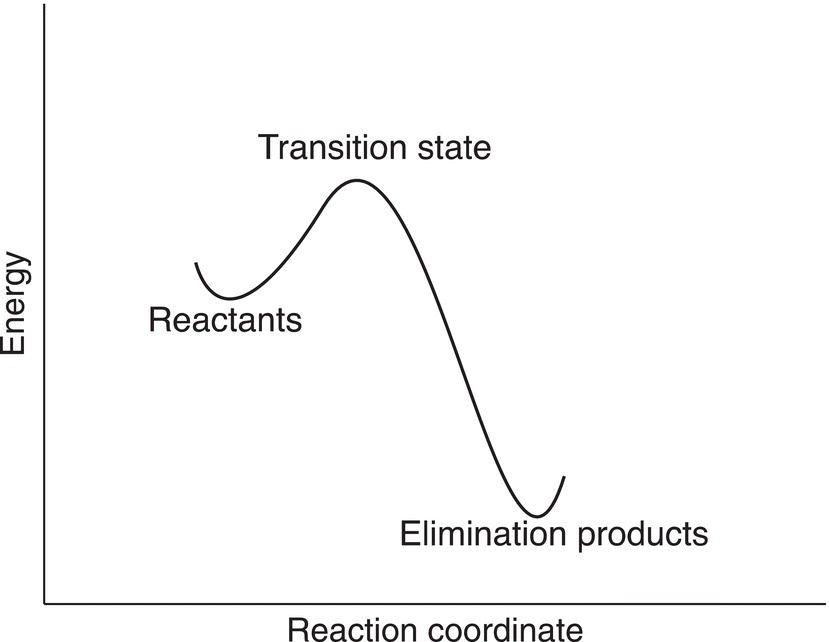
Figure 12.2 Energy profile of a β-elimination reaction. Note that this is a bimolecular single-step reaction (E2).
Shown below are two examples of β-elimination Reactions (12-2) and (12-3). Note that heat is typically applied for elimination reactions, and we will see later that this is one way to ensure that an elimination reaction takes place, compared to another type of reaction, substitution reaction, which will be covered in Chapter 15.
(12-2)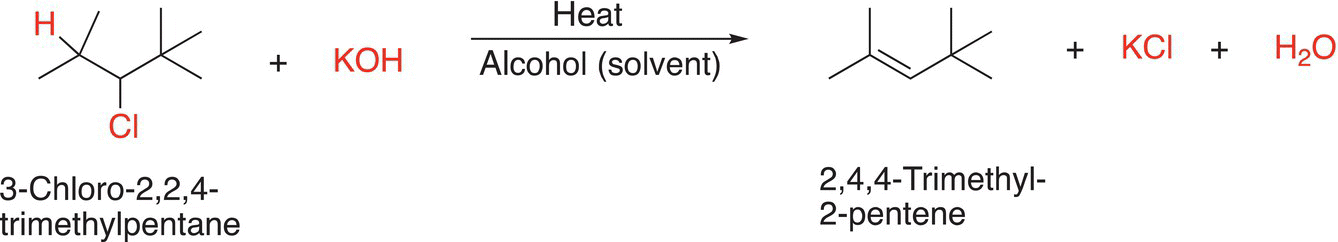
For the reaction shown in Reaction (12-2), the base, potassium hydroxide, abstracts the proton shown and as the leaving group, the chloride anion, leaves, the double bond is formed. Later in the chapter, we will examine other types of bases that can be used for these reactions. Note that the product shown is the only possible elimination product since there is not another hydrogen adjacent to the leaving chloride anion. The same is true for the reaction shown in 12-3. Later in the chapter, we will see that there are other possible elimination products.
(12-3)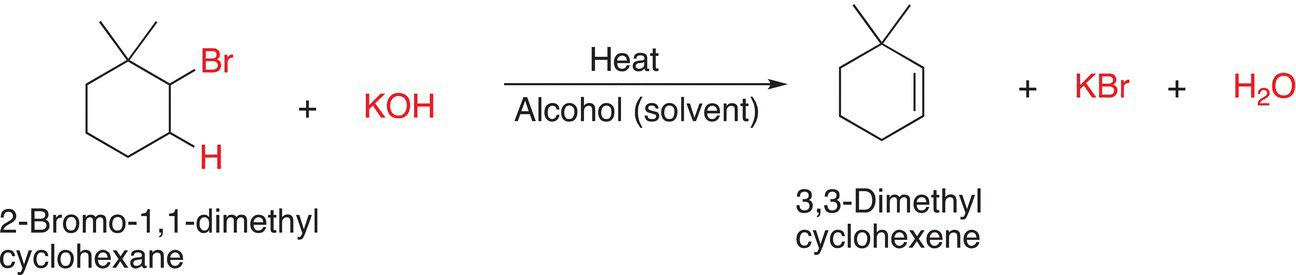
Another important feature of this mechanism for the elimination of a proton and a leaving group as shown in Figure 12.1 is that of the geometry; the hydrogen and the leaving group must be in the trans arrangement. Since the leaving group and hydrogen abstracted are typically opposite to each other, this type of elimination is also called a trans-elimination. For the elimination reaction shown in Reaction (12-4), note that the elimination takes place across the carbons that have the leaving group and the hydrogen that are in the trans arrangement. The other hydrogen that is adjacent to the leaving group is cis to the leaving group, hence elimination will not occur in that position.
(12-4)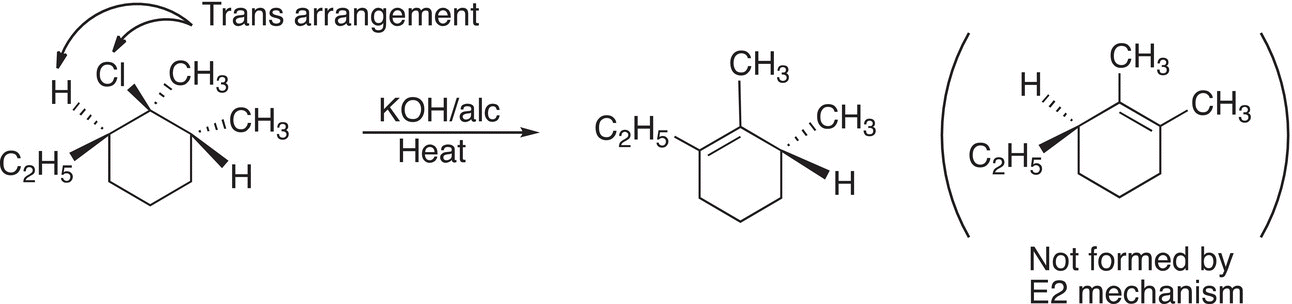
Note that if the stereochemistry of the leaving group and the hydrogen is not designated by the usual dashed-wedge or another method to show stereochemistry in the reactant, the elimination product could be from either side of the leaving group, as shown in Reaction (12-5).
(12-5)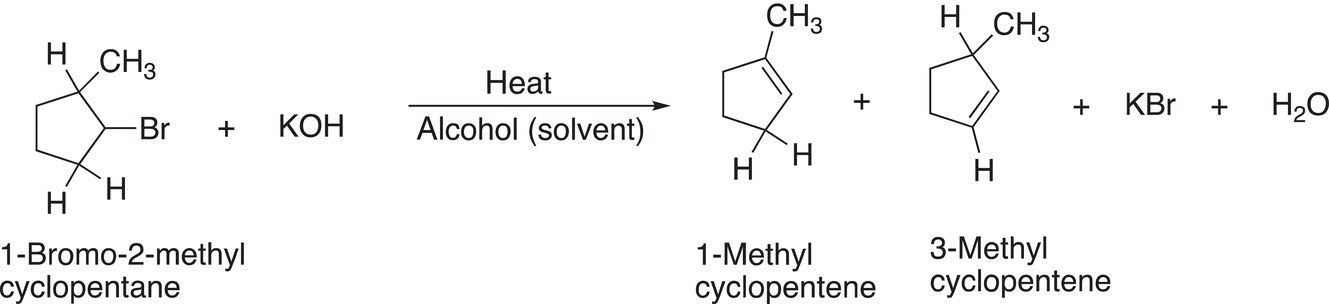
Note for alkyl halides that have a hydrogen on either of the carbons that are adjacent to the carbon that has the leaving group, there are two possible products, in which the double bonds are in different locations from the leaving group as shown in Reaction (12-5).
Problem 12.1
Give the elimination product for the reaction of KOH/alcohol at elevated temperature with each of the following alkyl halides. Note for some, it is possible to have more than one product, in which the double bonds are in different locations from the leaving group. Give all possible products.
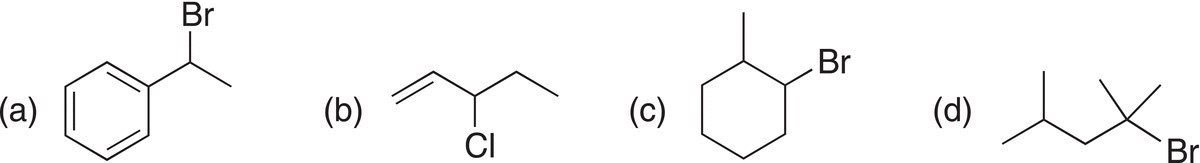
As shown in Reaction (12-5) and Problem 12.1, it is possible to abstract a proton from either carbon that is adjacent to the leaving group, which results in two different alkenes. Experimental conditions can be designed so that one alkene is the major product and the other is the minor product.
The outcome of getting one alkene as the major alkene product is dependent on two aspects: the size of the base and second the number and size of the groups surrounding the proton to be abstracted. As shown in Reaction (12-6), if there is steric bulk around the hydrogen to be extracted as a proton, it is extremely difficult for the base to accomplish that task.
(12-6)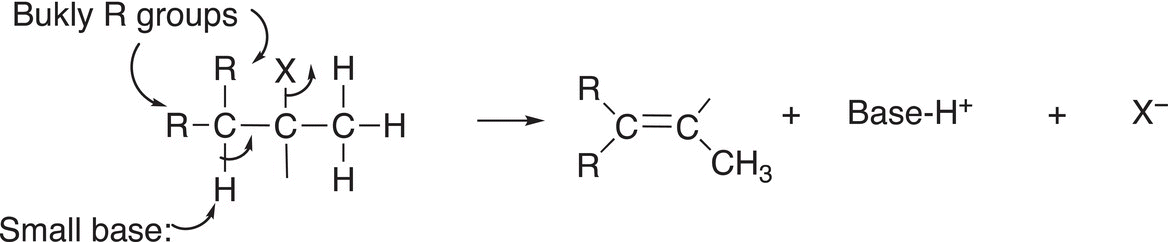
This does not mean that the abstraction will not take place, however, but it would require a base small enough (hard base) to accomplish the abstraction. A small base such as sodium hydroxide (NaOH) can accomplish this task. You may ask at this point, why wouldn’t the base abstract the adjacent proton instead since it is more accessible? The challenge is that the product that results from the abstraction of the proton from the carbon that has more steric bulk results in a more stable alkene. We have demonstrated in Chapter 4 that alkenes that have more alkyl groups surrounding the double bond are more stable than alkenes that have less alkyl groups surrounding the double bond.
On the other hand, if a very bulky base, such as lithium diisopropylamide (LDA) or potassium tert-butoxide, is used for the reaction, either of these bulky bases will not be able to abstract the proton from the carbon that has a lot of surrounding steric bulk. You will recall that we discussed these types of bases in the chapter on acids and bases (Chapter 7). As a result, it will abstract a proton from the carbon that has less steric bulk, i.e. from the carbon that has more hydrogen atoms owing to the very small size of the hydrogen atom. This concept is illustrated in Reaction (12-7).
(12-7)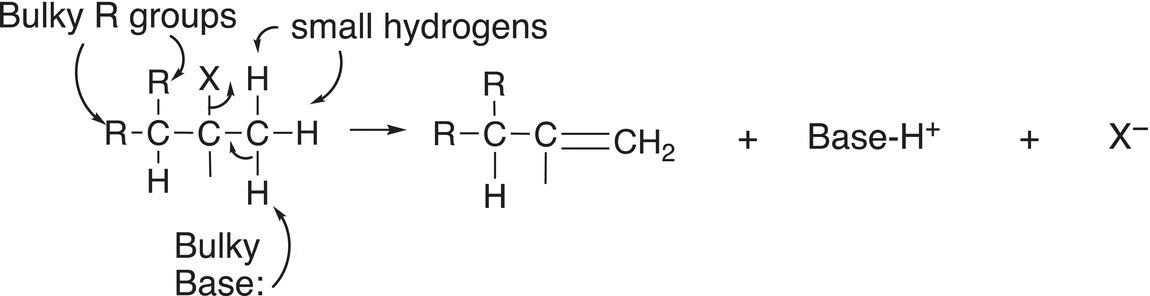
Note in this case, the product formed is the least stable since the double bond has less alkyl groups surrounding the double bond, compared to the alkene formed in the example shown in Reaction (12-6). Thus, it is possible for a reactant to form either of the two different major alkene products depending on the reaction conditions. The less substituted alkene product is often referred to as the Hofmann product and the more substituted alkene product as the Zaytsev product. This concept is summarized in Reaction (12-8).
(12-8)∆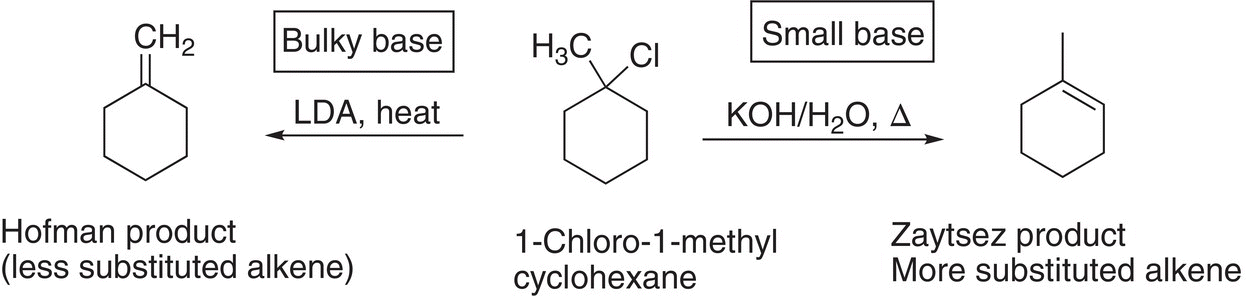
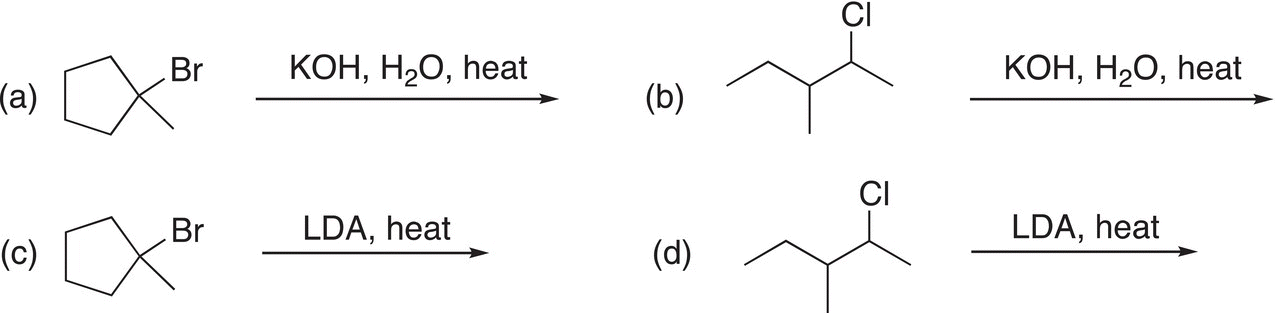
Problem 12.2
Give the major organic products for the following elimination reactions. Pay special attention to the size of the base used; KOH is a small base, whereas LDA is a bulky base.
Another approach that can be used to obtain the less stable alkene is instead of using a bulky base is to have the leaving group be a bulky leaving group. The outcome again depends on the ability of the base to access the acidic hydrogen for the elimination to occur. Reaction (12-9) shows the elimination reaction in which the bulky trimethyl ammonium ion is the leaving group; hence, the use of a small base, such as the hydroxide anion, can be used to carry out the elimination to give the least stable alkene as shown in Reaction (12-9).
(12-9)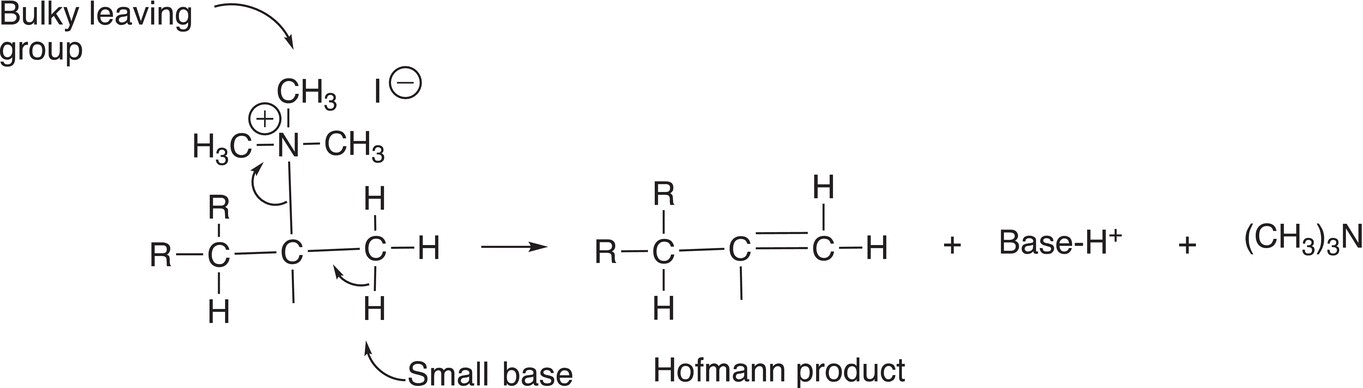
This type of reactant gives the product with the less substituted double bond, and is often referred to as the Hoffman elimination, an example is shown in Reaction (12-10).
(12-10)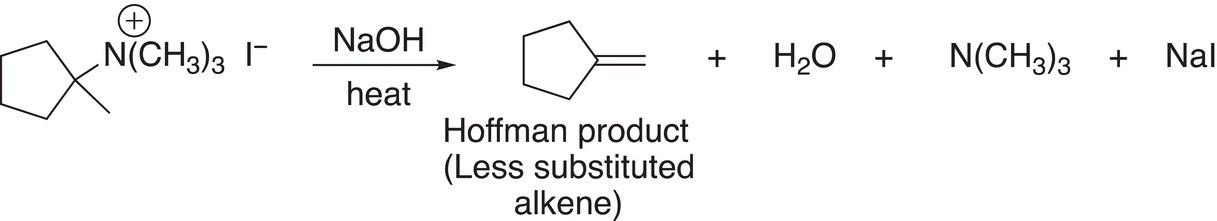
Problem 12.3
Give the Hofmann elimination product for each of the following compounds.
As you can imagine, it is possible to have an intramolecular elimination where the base and the leaving group are on the same molecule. This type of reaction is shown in Reaction (12-11).
(12-11)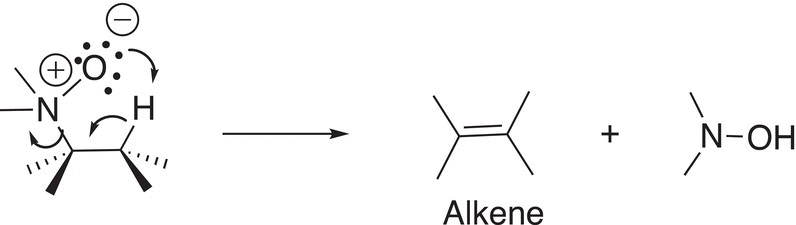
The N-oxide can be made from an oxidation reaction of an amine as shown in Reaction (12-12).
(12-12)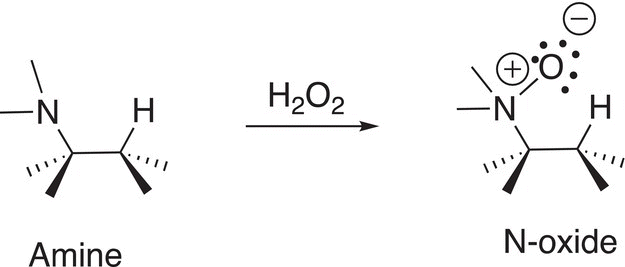
12.2.2 Elimination Unimolecular (E1) Reaction Mechanism
As mentioned earlier, there is an alternate mechanism by which elimination reactions can occur. During the course of an elimination reaction, the leaving group may leave with no influence from the base or another molecule. You will recall from Chapter 7 that very stable conjugate bases are good leaving groups. Thus, for a reaction to proceed by this mechanism, the leaving groups are typically very good leaving groups (stable conjugate bases), as illustrated in Reaction (12-13).
(12-13)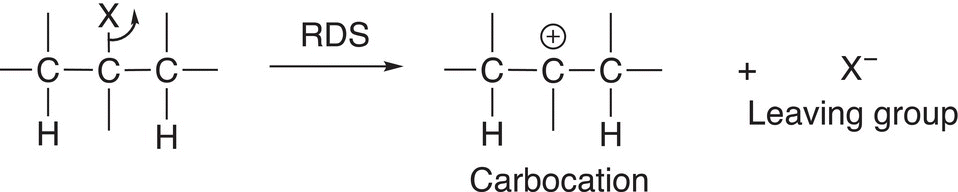
In a second step, the base removes a proton to form the alkene, which is shown in Reaction (12-14).
(12-14)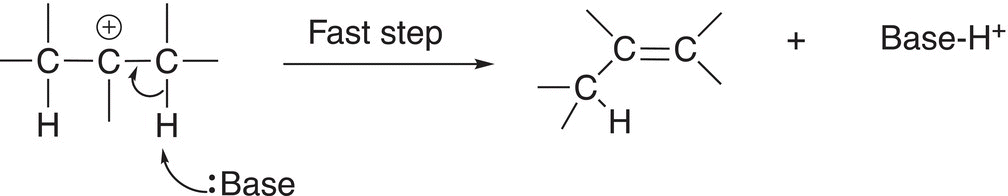
Note that if there is a hydrogen on the adjacent carbon of the carbocation, the double bond can be formed to the adjacent carbon as shown in Reaction (12-15).
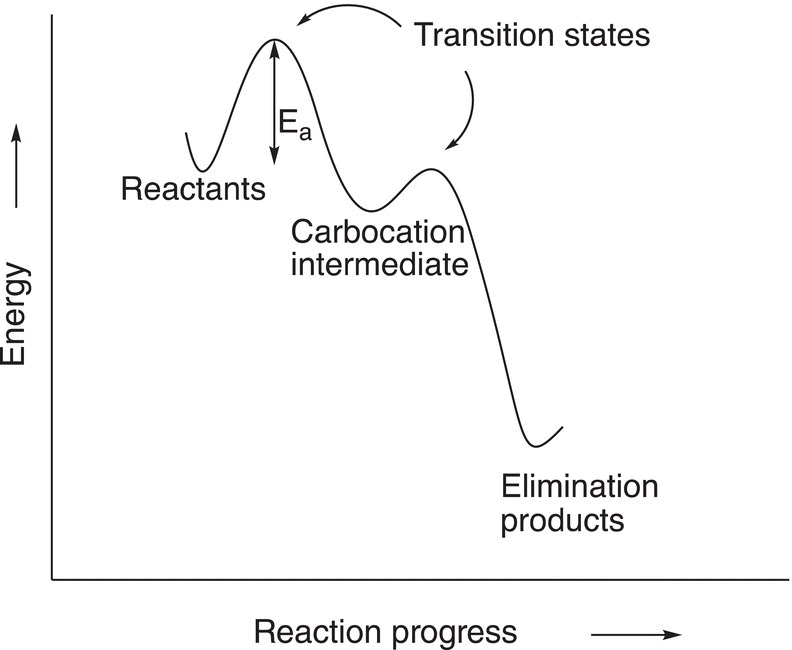
Figure 12.3 Energy profile of E1 reaction. Note that first step is the slowest step (the RDS) of the reaction mechanism (higher activation barrier (Ea), compared to the second step, which is fast.
(12-15)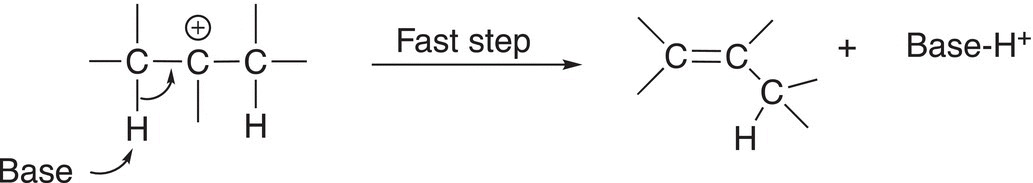
The formation of the carbocation in the first step of the reaction mechanism is the slowest and most important step of the reaction mechanism since it determines the overall rate of the reaction. Hence, this step has a special name, the rate-determining step (RDS), or rate-limiting step. Since only one reactant is involved in the most important step of this reaction mechanism, this mechanism is classified as a unimolecular elimination reaction (E1). The two steps of the reaction mechanism given in Reactions (12-14) and (12-15) can be represented by a reaction profile showing the two steps of the overall reaction, in which the first step has a higher activation barrier and hence slower, compared to the last step, which is fast. The reaction profile for this two-step reaction given in Figure 12.3 is different from the one-step reaction shown for the bimolecular E2 reaction shown earlier Figure 12.2.
12.2.3 Elimination Unimolecular — Conjugate Base (E1cB) Reaction Mechanism
A third possibility for elimination reactions occurs if the leaving group is a poor leaving group (relatively strong conjugate base), such as a hydroxyl or alkoxide group. For such reactions, which are carried out typically in a very basic medium, the first step involves the base abstracting a relatively acidic proton from the reactant to form a stable conjugate base intermediate as shown in Reaction (12-16).
(12-16)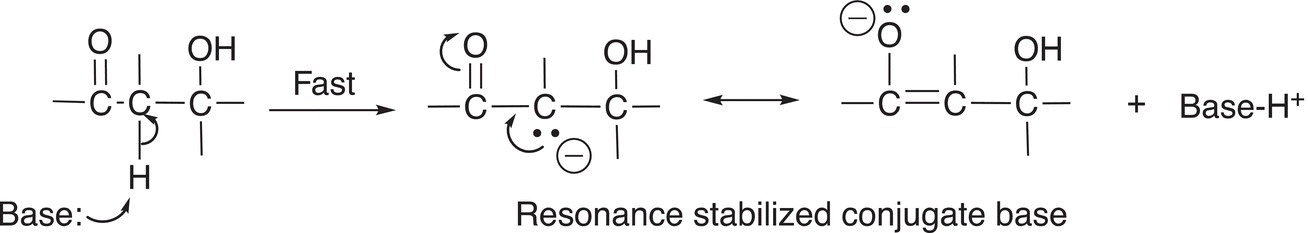
In a second and concerted step, the lone pair of electrons of the conjugate base folds in to form the double bond at the same time that the leaving group leaves as shown in Reaction (12-17).
(12-17)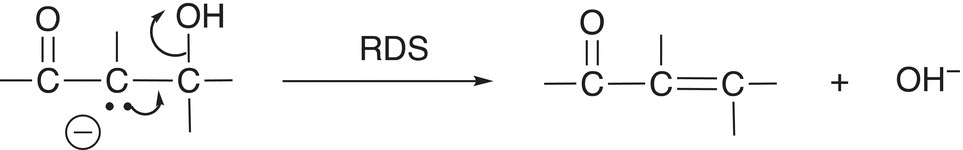
Since the leaving group is typically poor, these reactions are carried out at elevated temperatures. A specific example of a reaction that proceeds via an E1cB is shown in Reaction (12-18).
(12-18)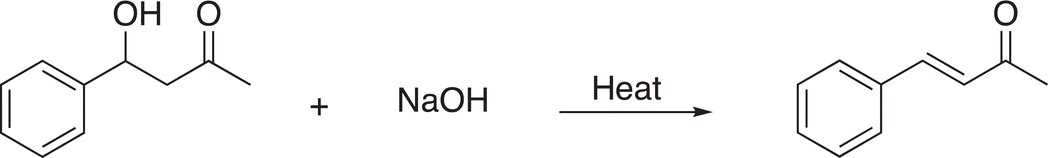
In this course, however, we will concentrate mostly on elimination reactions that proceed via E2 and E1 mechanisms and not much on E1cB reactions.