Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Spectroscopy Revisited, A More Detailed Examination
13.2 The Electromagnetic Spectrum
Energy can be described as massless photons traveling from one point to another at the speed of light, and the travel can be described in the form of waves. In this section, a detailed review of the electromagnetic spectrum will be carried out to ensure that students appreciate the amount of energy transmitted at the various regions of the electromagnetic spectrum. Energy travels at the speed of light and characteristics, such as wavelength and frequencies, can be used to describe the energy at various regions of the electromagnetic spectrum. Wavelength (λ) is the distance from one peak (or valley) of a wave to the next as shown in Figure 13.1. This distance is typically reported in centimeters (cm).
Figure 13.1 Representation of energy in the form of a wave.
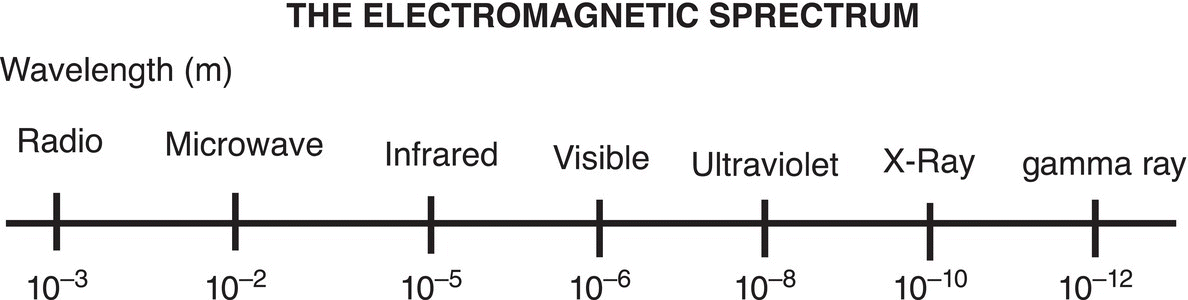
Figure 13.2 Relationship between the wavelength and the different regions of the electromagnetic spectrum.
The relationship between wavelength and the various regions of the electromagnetic spectrum is shown in Figure 13.2.
There is a mathematical equation that relates the wavelength to the energy of the different regions of the electromagnetic spectrum and is given in Eq. (13-1).
(13-1)![]()
In Eq. (13-1), ΔE is the relative energy, h is a constant (Planck's constant), c is the velocity of light, and λ is the wave length; note that the energy is inversely proportional to wavelength (λ). That is, if the wave length is short, there is a lot of energy. Table 13.1 gives the relative energies and the wavelength ranges for the various regions of the electromagnetic spectrum as well as descriptions of different regions and their effects on molecules.
Based on this information, gamma rays are the most dangerous. Another characteristic of energy is the frequency, which is the number of cycles that passes a specific point in a given time, typically in a second and is usually represented by the Greek letter ν and given in hertz (Hz). Equation (13-2) gives the relationship between frequency and energy.
(13-2)![]()
From the relationship in Eq. (13-2), note that energy is directly proportional to the frequency.
The wave number (v˜) is another variable that is used to represent energy and the units are in reciprocal centimeter, cm−1. Equation (13-3) gives the relationships with energy and various variables, note that the energy is directly proportional to the wave number and inversely proportional to the wavelength.
(13-3)![]()
Table 13.1 Relationship between different regions of the electromagnetic spectrum, their energies and effects on molecules.
Wavelength, λ (cm) |
Region of EM |
Energy (kJ mol−1) |
Effect of molecule |
10−9 |
Gamma (γ) rays |
107 |
Ionization |
10−7 |
X-rays |
105 |
Ionization |
10−7 |
Vacuum ultraviolet |
103 |
Ionization |
10− |
Near ultraviolet |
103 |
Electronic transition |
10−4 |
Visible |
102 |
Electronic transition |
10−3 |
Infrared |
10 |
Molecular vibrations |
10−1 |
Microwave |
10−2 |
Molecular rotations |
102—104 |
Radio waves |
10−4—10−6 |
Nuclear spin transition |
13.2.1 Types of Spectroscopy Used in Organic Chemistry
There are different types of spectroscopy that are used for the determination of the presence of different functional groups and the structure of compounds. Spectroscopy involves the interpretation of the various ways that molecules interact with different forms of energy that are described in Table 13.1. The energy used in organic spectroscopy is from different regions of the electromagnetic spectrum, which include visible light, ultraviolet light, microwave, IR, and radio waves. Since each molecule interacts differently in the presence of different types of energy, the nature of these interactions will give an idea of the structure of unknown compounds. Once energy of a specified frequency is exposed to molecules, an interaction occurs that causes the molecules to be in an excited state owing to the energy absorbed. Once this energy is absorbed, there are different outcomes. Thus, based on how molecules interact with energy of the various regions of the electromagnetic spectrum, that information can be used to assist in determining the identity of unknown molecules and identify the presence of different functional groups.
DID YOU KNOW?
When water molecules are excited by energy in the microwave region of the electromagnetic spectrum. Energy from the microwave oven causes polar water molecules to rotate and, as a result generate heat, which is used to heat our food in the microwave oven. You know from experience that if water is heated in a Styrofoam cup using the microwave oven, the cup is not heated, just the water. For the package of energy that is used in microwave ovens, water molecules are excited and not Styrofoam for example.
