Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Spectroscopy Revisited, A More Detailed Examination
13.3 UV-Vis Spectroscopy and Conjugated Systems
Let us start our discussion of UV-Vis spectroscopy by first examining the effects of energy on molecular orbitals of ethylene. You will recall from Chapter 1 that the two carbons of ethylene are both sp2 hybridized, which means that each carbon contains an unhybridized p orbital, which is perpendicular (orthogonal) to the plane of the atoms of the ethylene molecule. Each p orbital has one electron and these electrons form two molecular pi (π) orbitals, one lower in energy than the other. The representation is shown in Figure 13.3.
ΔG in Figure 13.3 is the energy required to promote one electron from the lower molecular orbital, also known as the highest occupied molecular orbital (HOMO), to the higher antibonding orbital, which is also known as the lowest unoccupied molecular orbital (LUMO). There is an exact amount of energy that is required for this electron promotion that can be expressed in terms of wavelength by the relationship that we saw earlier in the chapter. For this single isolated double bond, the exact wavelength (λ) is 165 nm as shown in Figure 13.4.
You will recall that this wavelength is in the region of the UV-Vis region of the electromagnetic spectrum. The instrument that is used to supply energy in this region of the electromagnetic spectrum is called a UV-Vis spectrophotometer. By scanning the UV-Vis region, a signal is obtained if a sample has a double bond that corresponds to this specific wavelength. Thus, if a molecule has an isolated double bond, you would expect a signal in the region of 165 nm of the UV-Vis spectrum. This type of information can be used to confirm that an unknown compound has a double bond if it has a signal in this region of the UV-Vis spectrum.
Let us now consider another system where there is another double bond next to an ethylene double bond, as in 1,3-butadiene. The pi (π) molecular orbitals are shown in Figure 13.5.
The energy gap ΔG between the HOMO and LUMO is smaller for 1,3-butadiene, compared to ethylene. This type of shift to a longer wavelength (less energy) is referred to a bathochromic shift. 1,3-Butadiene has two double bonds that are in conjugation with each other. The energy that is required for the promotion of an electron from the HOMO to the LUMO is in the UV-Vis range of the electromagnetic spectrum and the wavelength is 217 nm. The UV-Vis spectrum of isoprene, which has two double bonds in conjugation, is shown in Figure 13.6.
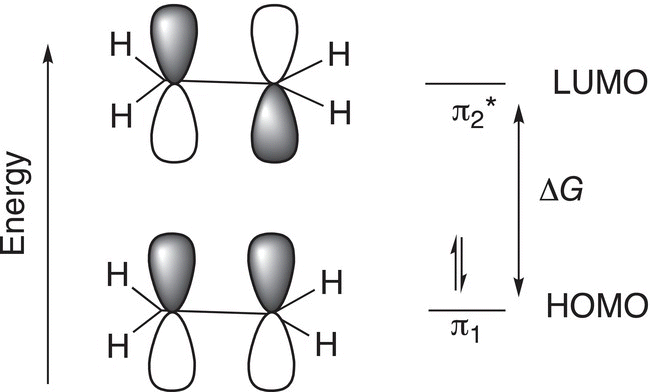
Figure 13.3 Ground state electronic configuration of ethylene showing the pi (π) molecular orbitals of ethylene.
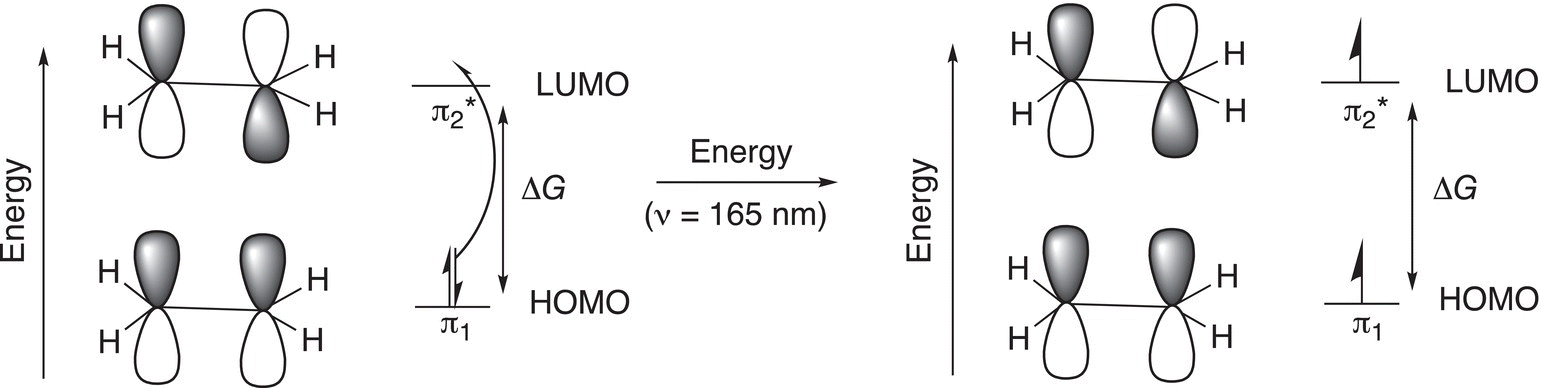
Figure 13.4 Ground state electronic configuration and excited state of ethylene showing the pi (π) molecular orbitals of ethylene after specific energy is absorbed.
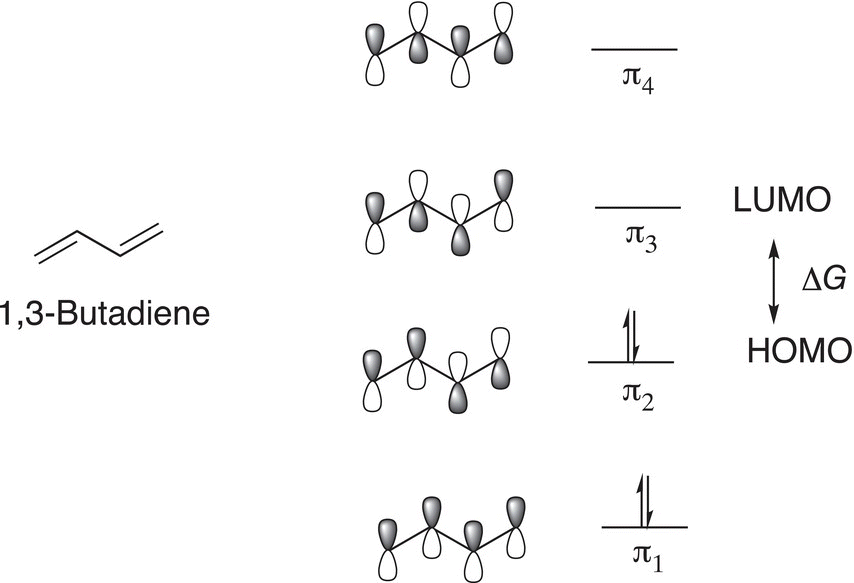
Figure 13.5 pi (π) orbitals of 1,3-butadiene.
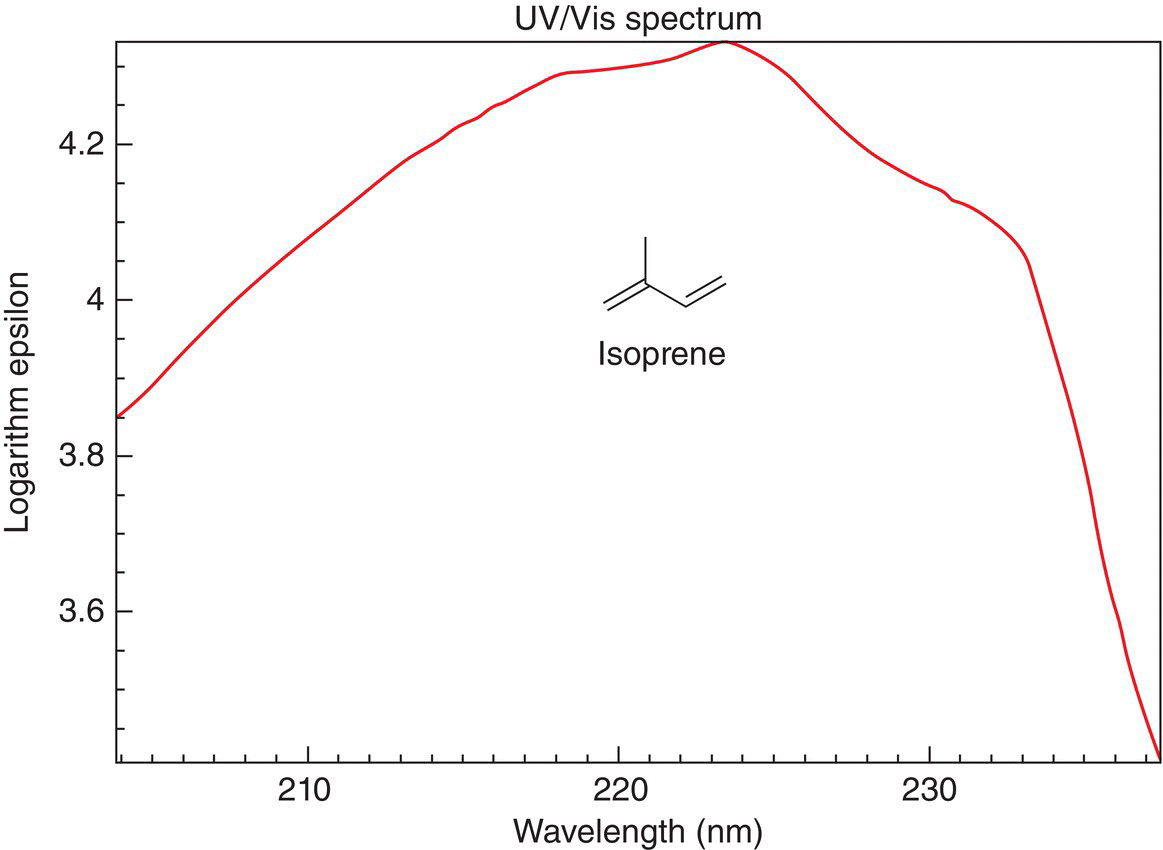
Figure 13.6 UV-Vis spectrum for isoprene, which has two double bonds in conjugation, λmax is 217 nm.
Source: with permission from NIST.
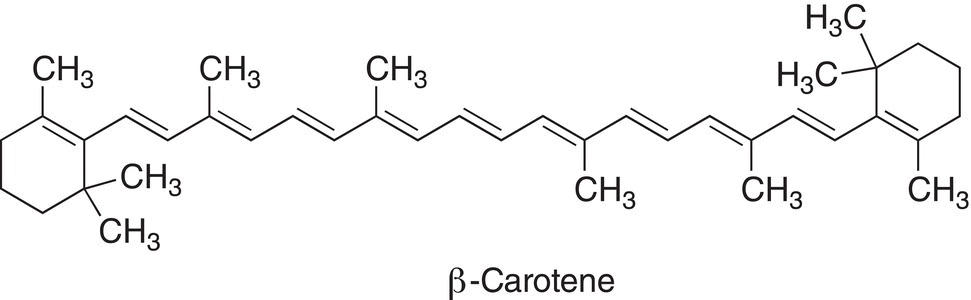
Notice that the energy requirement is now approximately 217 nm, compared to 165 nm of ethylene. If there were another double bond in conjugation as in 1,3,5-triene, the energy requirement for promotion would be less as reflected by the wavelength of approximately 258 nm. The human eye sees only the colors in the visible region of the electromagnetic spectrum. Thus, if there is a compound that absorbs strongly in this region, that color is absorbed in the electronic transition and what we see is the rainbow colors, minus that color. A large percentage of organic compounds contain various numbers of conjugated double bonds. β-Carotene, which is a highly colored compound, has 11 double bonds in conjugation; β-carotene is a precursor of vitamin A, which is needed for good health and vision. β-Carotene, which structure is shown below, absorbs at 454 nm (blue region), so its absorbance is in the visible region of the electromagnetic spectrum. The human eye detects the β-carotene in carrots as orange since that energy at that wavelength is absorbed in the transition and the human eye sees the remainder of colors.
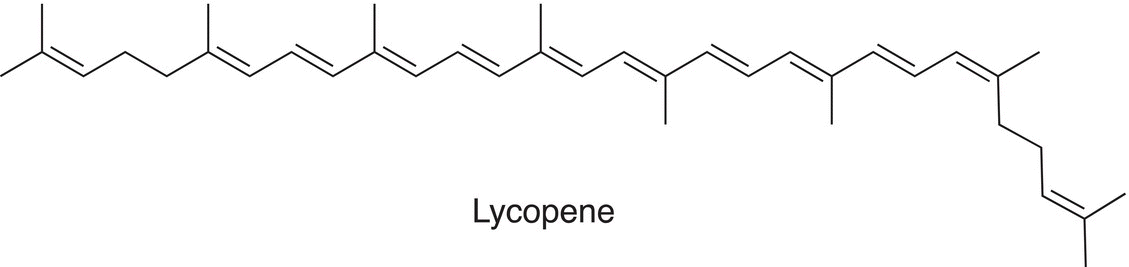
Another compound with extended conjugation is lycopene, which is a bright red carotene. Carotenoid pigment and other phytochemicals are found in tomatoes and other red fruits and vegetables. The λmax for lycopene is 474 nm. As you will conclude from these spectra, the energy requirement for the transition in the UV-Vis is lower for extended conjugation.
DID YOU KNOW?
Some of the constituents of fruits and vegetables have photochemicals that have different numbers of double bonds that are conjugated and give rise to their different colors. The bright colors of carrots and tomatoes are due to the presence of highly conjugated systems, β-carotene and lycopene, respectively. The human eye sees only the colors in the visible region of the electromagnetic spectrum and if there is a compound that absorbs strongly in this region, that color is absorbed in the electronic transition and what we see is the rainbow colors, minus that color. β-Carotene absorbs at 454 nm, and lycopene absorbs at 471 nm in the region of the electromagnetic spectrum.
Conjugation may also involve unshared electrons on an atom adjacent to double bonds. Curcumin and Ruhemann's purple both have extended conjugations that involve a pair of nonbonding electrons of the oxygen atoms.
Curcumin is a compound that is found in turmeric, which is a well-known spice. Ruhemann's purple is produced in the detection of fingerprints as we will see in more detail in Chapter 20 on the section of peptides and amino acids. As you can imagine, there are ways to calculate the λmax for different conjugated systems based on the number of double bonds in conjugation and the number and types of substitutions about the double bonds. The Wood—Fieser and Fieser—Kuhn rules can be used to get good estimates of λmax, but the details will not be discussed in this chapter, but are handled in more advanced spectroscopy courses. We should be able to estimate, however, the region of absorption for molecules with extended conjugated double bonds.
Problem 13.1
i. The structure of vitamin E is shown below; would you expect it to have a similar UV-Vis spectrum as β-carotene? Explain your answer.
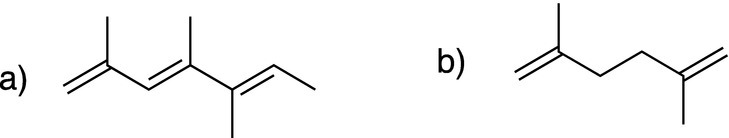
ii. Based on your knowledge of the UV-Vis spectra of ethylene and 1,3-butadiene, estimate if the maximum absorption of the molecules shown below is greater than or less than that of ethylene.