Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Spectroscopy Revisited, A More Detailed Examination
End of Chapter Problems
1. 13.7 The following reaction was carried out, determine how would you use spectroscopy to determine if there was complete conversion to product?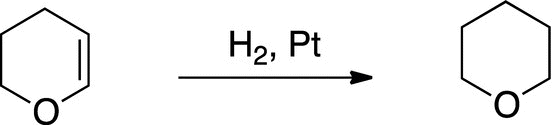
2. 13.8 An unknown compound showed a molecular weight ion peak at 134.2 m/z. The 1H NMR and IR spectra are given below. Give a likely structure of this unknown compound.
3. 13.9 A tertiary alcohol (A) is treated with the strong base, sodium hydride, to form a salt, which then reacts with methyl bromide to form a new compound B. The mass spectrum for compound A has a M + 1 peak at m/z = 117 and other peaks at m/z = 87 and m/z = 73. Propose structures for compounds A and B.
4. 13.10 The hydration of 2-pentene gave an unknown alcohol. Give a brief description of how you could use spectroscopy to determine the structure of the hydration product.
5. 13.11 The NMR of N,N-dimethylacetamide (structure shown below) consists of three singlets at δ = 2.1, δ = 2.93, and δ = 3.03. If the temperature should increase, the NMR spectrum of this compound changes slightly resulting in just two signals: δ = 2.1 and δ = 2.98 with the signals at δ = 2.93 and δ = 3.03 coalesce into the signal at 2.98 with twice the intensity. Explain this observation.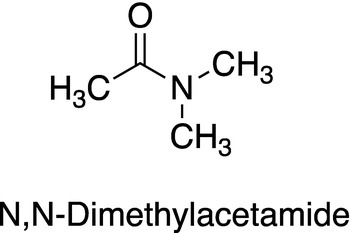
6. 13.12 The spectra for an unknown compound are given below. Based on the spectra provided, determine the structure of the unknown compound.