Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Free Radical Substitution Reactions Involving Alkanes
14.2 Types of Alkanes and Alkyl Halides
14.2.1 Classifications of Hydrocarbons
As pointed out in Chapter 4, hydrocarbons, and especially alkanes, are the source for many organic compounds, including alkyl halides. There are literally thousands of different alkanes and they can be categorized into four common categories based on the number of hydrogens and carbons that are bonded to a specific carbon, as shown below (Figure 14.2).
In the first example, the methyl carbon, and hence methyl hydrogen, is derived from methane since there is only one carbon atom involved. The primary carbon is bonded to one alkyl group (R) and hence the hydrogens bonded to that carbon are described as primary hydrogens; the symbol that is used to represent a primary carbon and primary hydrogens is 1°. The secondary carbon is bonded to two alkyl groups and hence the hydrogens that are bonded to that carbon are called secondary hydrogens the symbol that is used to represent a secondary carbon and secondary hydrogens is 2°. The tertiary carbon is bonded to three R groups and has only one hydrogen bonded to that carbon; the hydrogen that is bonded to this carbon is classified as a tertiary hydrogen; the symbol that is used to represent a tertiary carbon and tertiary hydrogens is 3°.
Let us now apply that classification to propane, which has different types of hydrogens and carbons. Propane has two types of hydrogens, primary and secondary hydrogens, as shown in Figure 14.3.
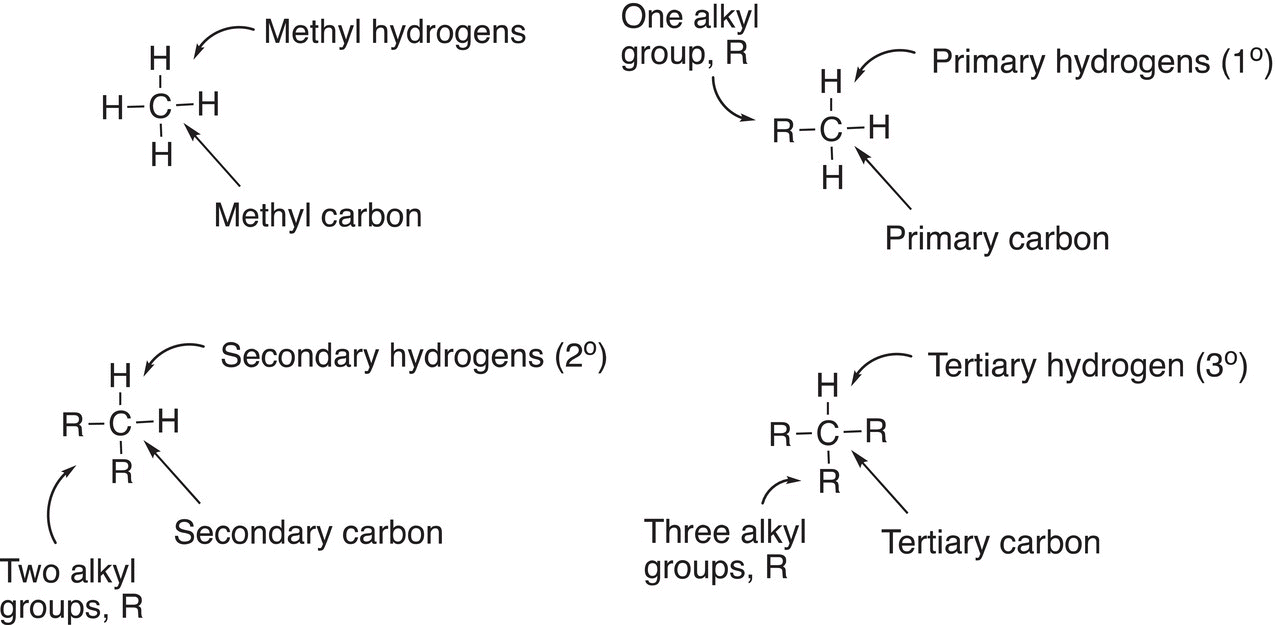
Figure 14.2 Types of carbons and hydrogens of alkanes.
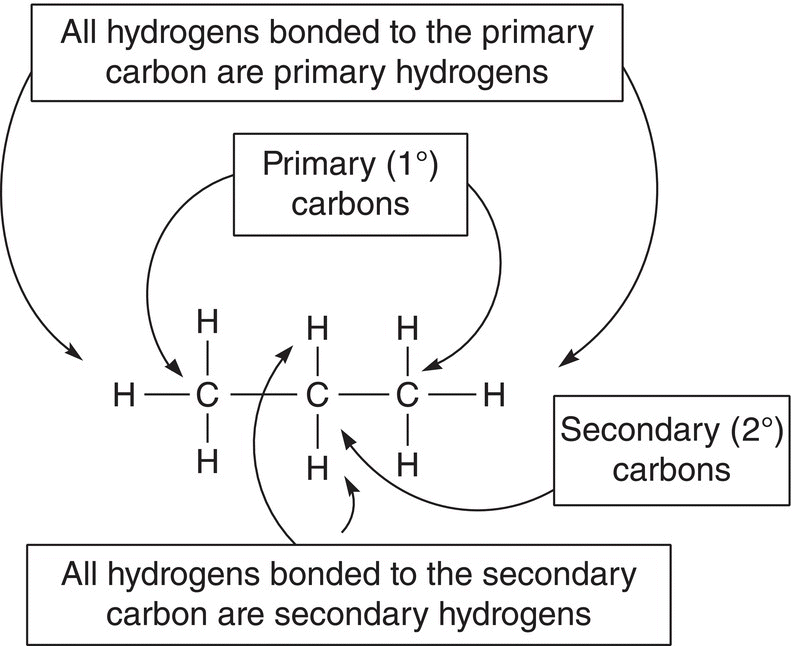
Figure 14.3 Types of carbons and hydrogens of propane.
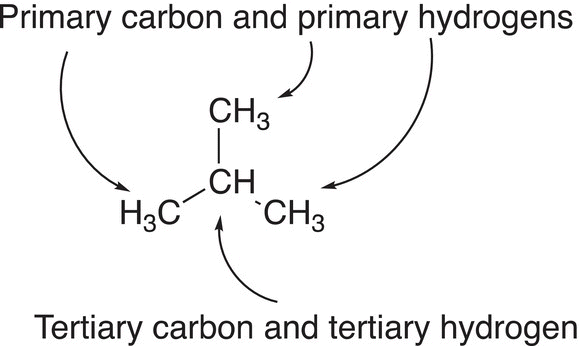
Figure 14.4 Types of hydrogens bonded to 2-methylpropane (isobutane).
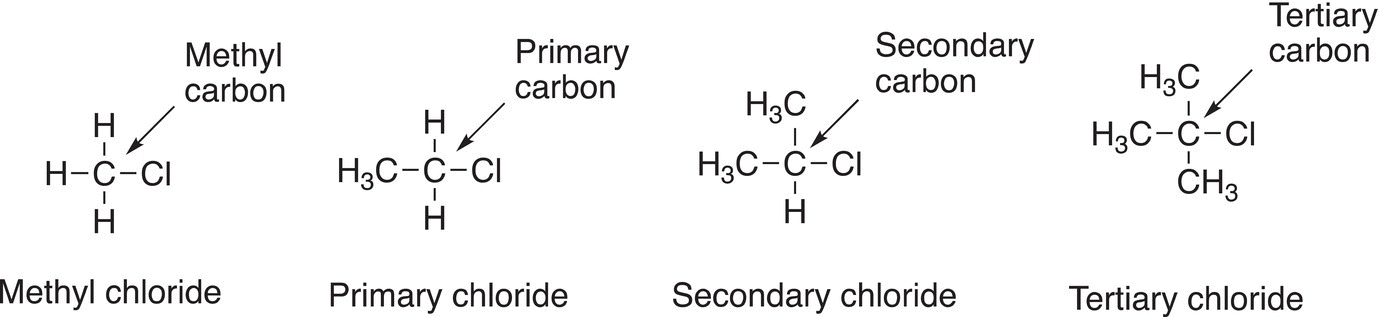
Figure 14.5 Examples of the classification of different types of alkyl chlorides.
Figure 14.4 gives another example showing the different types of carbons and hydrogens of 2-methylpropane (isobutane).
This same system of classification can be used to classify alkyl halides. The alkyl halides that will be encountered in this chapter can be classified as: methyl, primary, secondary, or tertiary halides. A methyl halide is an alkyl halide in which the halogen is bonded to a methyl carbon; a primary halide is an alkyl halide in which the halogen is bonded to a primary carbon; a secondary halide is an alkyl halide in which the halogen is bonded to a secondary carbon; and a tertiary halide is one in which the halogen is bonded to a tertiary carbon. Examples of the types of alkyl halides are shown in Figure 14.5.
Problem 14.1
i. Draw the expanded structure of the following alkanes and classify each hydrogen as 1°, 2°, or 3°.
1. 2-Methylbutane
2. 2,2-Dimethylpentane
3. Methylcyclopentane
ii. Classify the following alkyl halides as methyl, 1°, 2°, or 3°.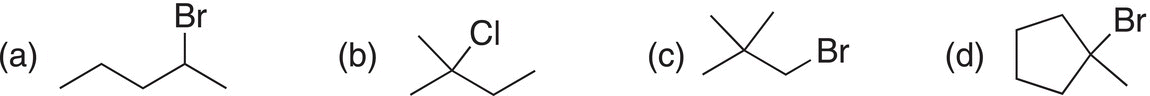
In addition to the primary, secondary, and tertiary classifications described above, there are other commonly used classifications, which are shown below.
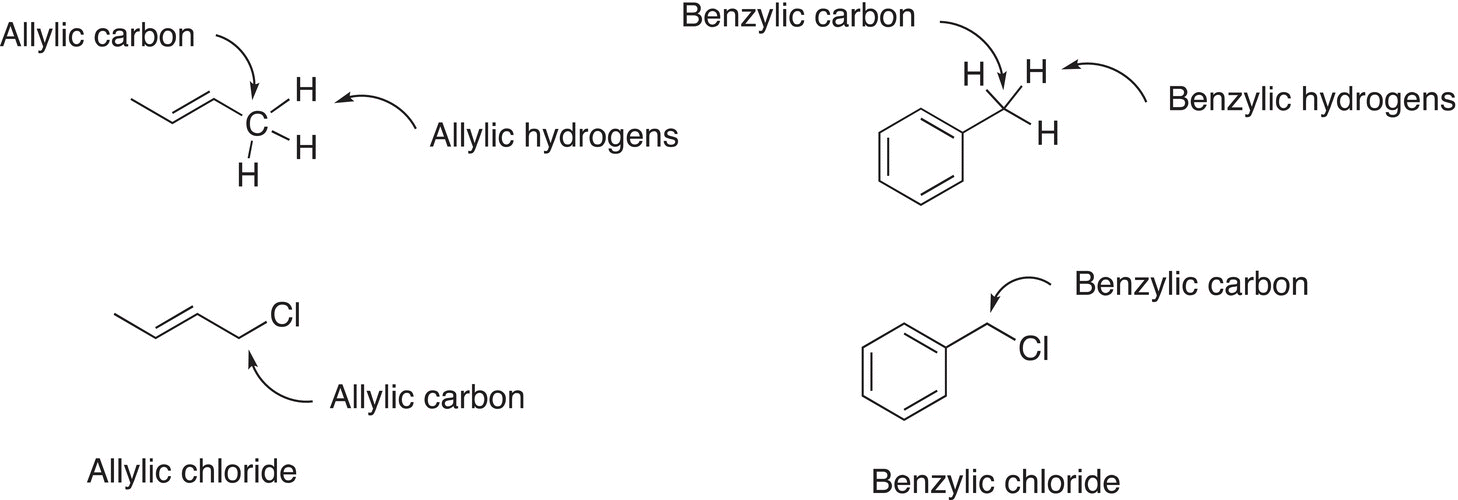
Table 14.1 Bond dissociation energies for different types of hydrocarbons.
Bond |
Energy (kcal mol−1) |
Type of C─H bond |
CH3─H |
105 |
Methyl |
CH3CH2─H |
98 |
Primary (1°) |
(CH3)2CH─H |
95 |
Secondary (2°) |
(CH3)3C─H |
93 |
Tertiary (3°) |
C6H5CH2─H |
90 |
Benzylic |
CH2═CHCH2─H |
89 |
Allylic |
A major difference between the allylic and benzylic system of classification is the carbon adjacent to the carbon being classified; an allylic carbon is adjacent to a carbon—carbon double bond, and a benzylic is adjacent to a benzene ring.
14.2.2 Bond Dissociation Energies of Hydrocarbons
The bond strengths of the C─H covalent bond for the different types of hydrocarbons discussed in the previous section are different. Some require more energy to break in a homolytic manner, compared to others that require less energy. Table 14.1 gives the bond dissociation energies for different types of C─H bonds.
Note that it requires more energy to break a methyl C─H bond, compared to a primary C─H bond. Likewise, it is easier to break an allylic or benzylic C─H bond, compared to a secondary or tertiary C─H bond. This concept will be very important when we start our analysis of reactions of alkanes. Now that we have examined the covalent bond, let us look at some of the properties of this type of bond. The typical covalent bond length is about 0.74 Å to about 2.0 Å. Short covalent bonds are typically stronger than longer covalent bonds. Triple bonds are short and strong, while single bonds are longer and weaker than triple bonds. It is possible to supply enough energy to break covalent bonds, especially a single bond so that the cleavage results in an equal distribution of the bonding electrons between the two atoms of that bond. The energy needed to break covalent bonds is called the bond dissociation energy. There are two possible ways in which a single covalent bond can be broken. Since there are two electrons in a single covalent bond, it is possible to break such a bond so that each atom of the bond gets one electron each. This type of bond cleavage is called a homolytic cleavage. The species that are produced in this case are called radicals. For the homolytic cleavage of hydrogen molecule, the amount of energy that must be supplied to break one mole of the hydrogen—hydrogen bond of the hydrogen molecule is 104 kcal, i.e. the bond dissociation energy is 104 kcal mol−1 as shown in Reaction (14-1).
(14-1)∆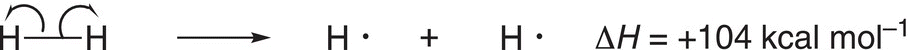
Note that a single-barbed arrow is used to represent the movement of one electron for the bond breakage and that the value of the dissociation energy is positive since energy must be supplied in order to break the bond. Thus, the formation of one mole of hydrogen gas from the atoms will liberate the same quantity of energy as shown in Reaction (14-2).
(14-2)∆
The same analysis can be applied to the bonds of hydrocarbons, as shown in Reactions (14-3) and (14-4).
(14-3)∆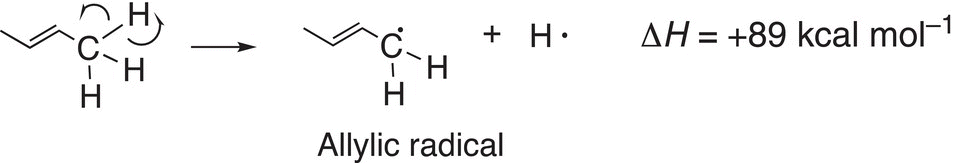
(14-4)∆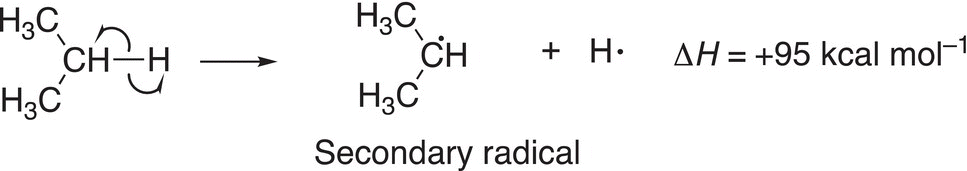
The homolytic cleavage of the allylic C─H bond results in a allylic radical and a hydrogen atom, whereas the homolytic cleavage of the secondary C─H bond results in a secondary radical and a hydrogen atom. Based on the bond dissociation energy values, it requires more energy to cleave the secondary C─H bond, compared to the allylic C─H bond. One conclusion that can be drawn from this observation is that the allylic radical is more stable than the secondary radical since reactions tend to proceed to give the more stable product or intermediate which would require the least amount of energy. Figure 14.6 gives an illustration of this concept.
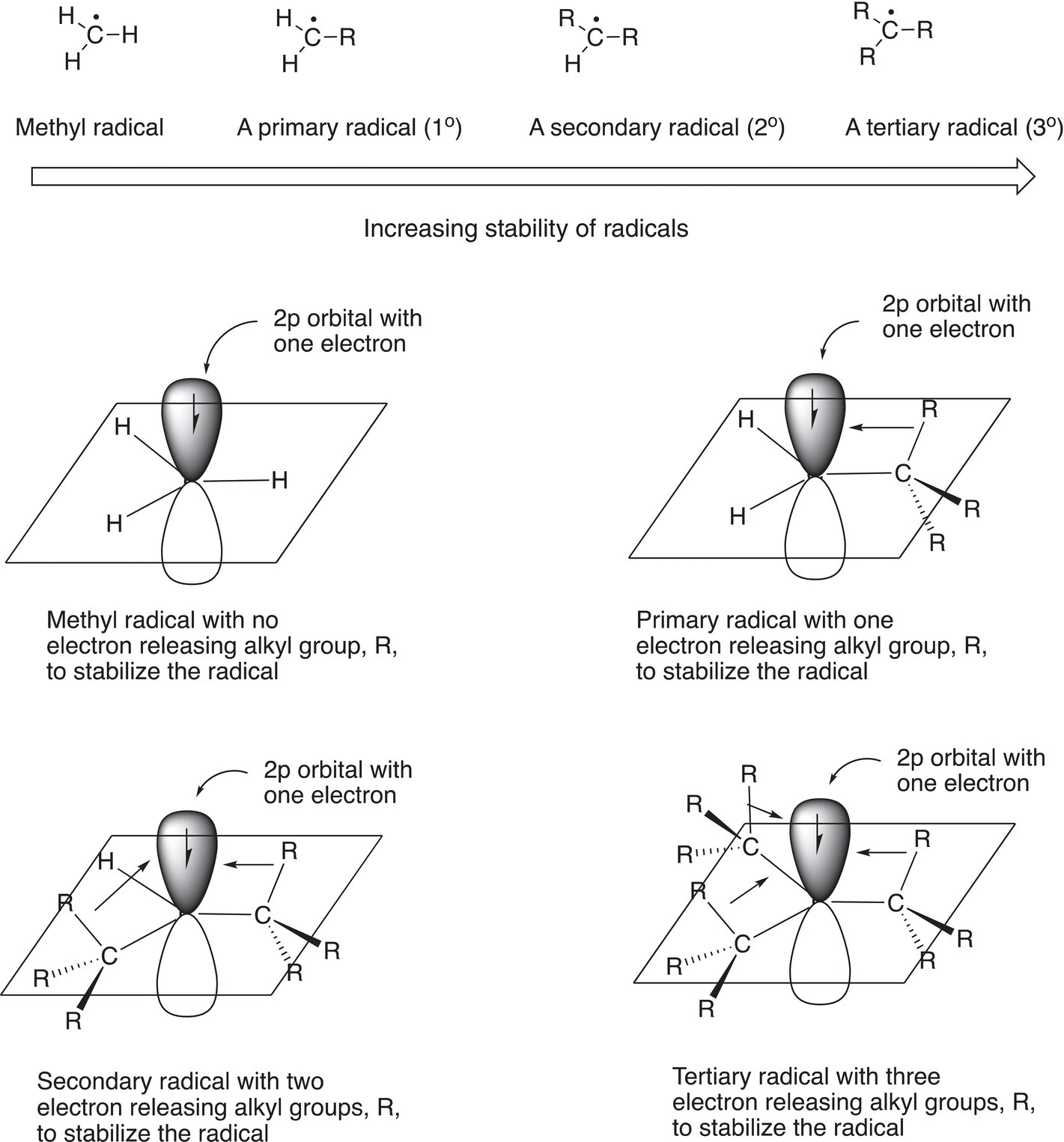
14.2.3 Structure and Stability of Radicals
Since radicals contain a single electron in the p orbital, it would much prefer to have another electron to have a full orbital. If there are electrons surrounding the radical, this will provide some degree of stability to the radical. Obviously, the more electrons in the vicinity of the radical, the more stable will be the radical. We have seen a similar situation when we examined carbocation. More alkyl groups around a radical provide stability to the radicals. Hence, similarly the stability trend for radicals is shown below.
∆∆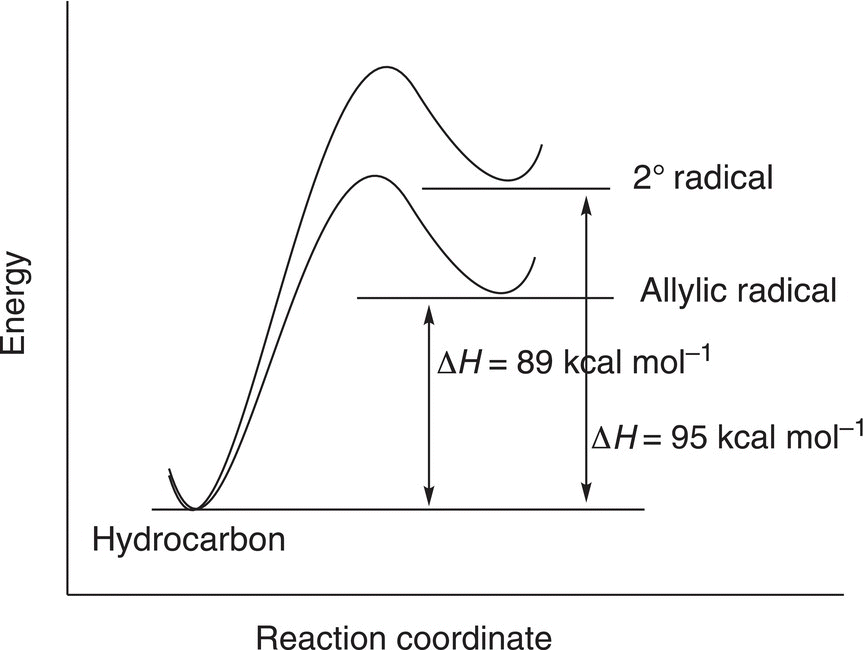
Figure 14.6 Relative energies showing the difference in energies for different types of radicals.
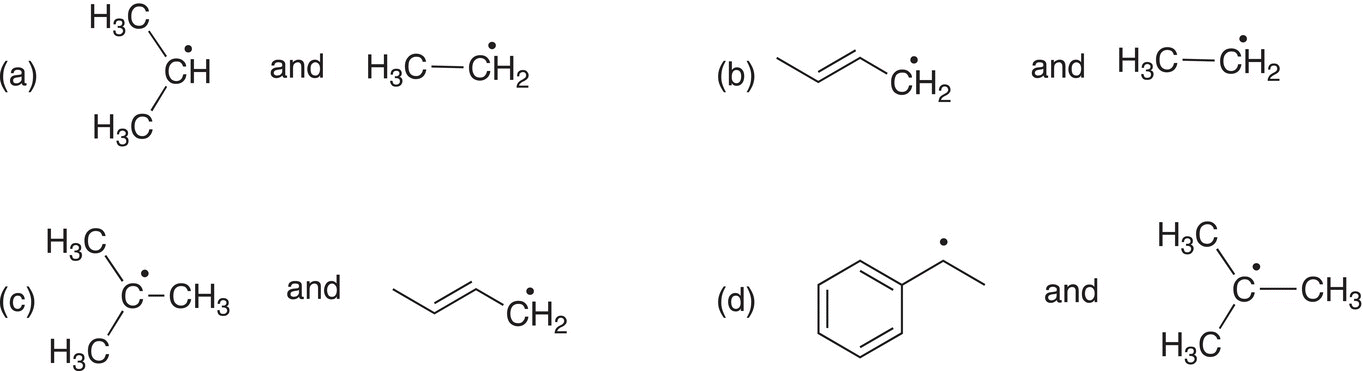
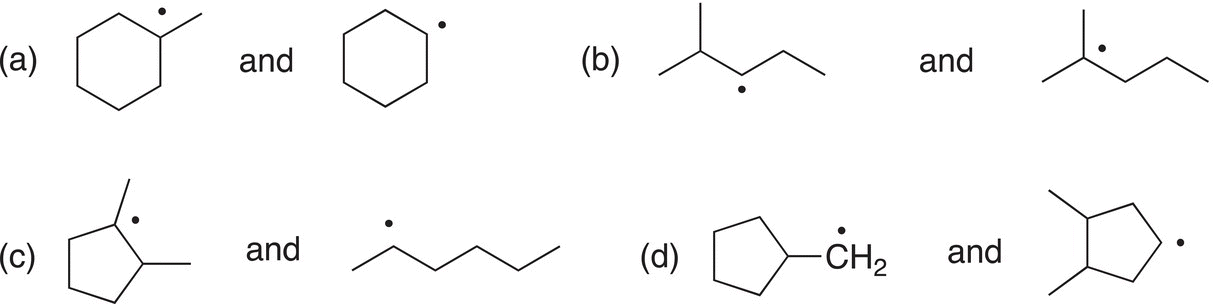
Problem 14.2
Of the following pairs of radicals, determine which is more stable?
A radical that is adjacent to a carbon—carbon double bond is called an allylic radical as we have seen in the previous section. Allylic radicals are stable due to a flow of electrons, which are located in the adjacent orbitals into the singly occupied p orbital of the radical. As a result of such electron flow, an allylic radical is more stable than a tertiary radical; a similar case exists for benzylic radicals. This concept of electron flow in the stabilization of allylic and benzylic radicals can be illustrated by resonance structures as shown below.
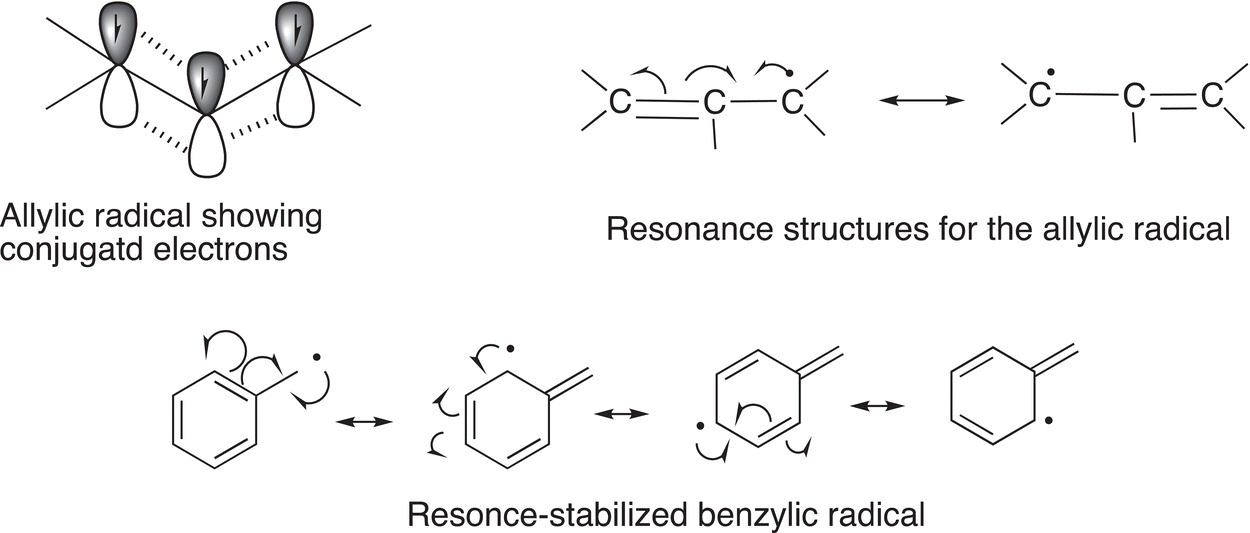

Figure 14.7 Order of stability of radicals.
Thus, the relative magnitudes of the bond dissociation energies can be used to determine the relative stabilities of different types of radicals, and the order is shown in Figure 14.7.
Problem 14.3
Of the following pairs of radicals, determine which of the following radicals is more stable?