Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Free Radical Substitution Reactions Involving Alkanes
14.4 Bromination of Alkanes
A major observation for the chlorination of alkanes is that there is almost an equal mixture of different mono-chlorination organic products obtained where the alkane has different types of hydrogens. Thus, if we were trying to get a high yield of a particular halogenated product using an alkane as a starting compound, chlorination would not be the ideal reaction to use in order to give a specific halogenated product as the major organic product in high yield.
14.4.1 Bromination of Propane and Other Alkanes
It is known from various experiments that if bromine is used for the same type of free radical substitution reaction instead of chlorine, the outcome is very different, as shown in Reaction (14-20), compared with Reaction (14-17).
(14-20)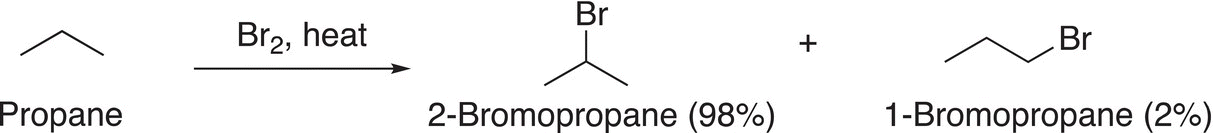
From Reaction (14-20), it is obvious that utilizing bromine gives an extremely high percentage of one halogenated product (2-bromopropane) compared to the other halogenated product (1-bromopropane). You will also notice that compared to the chlorination products, there is a drastic difference in the distribution of the halogenated products. In order to explain this difference, we will have to examine the mechanism for this type of free radical substitution reaction again. In the first step of the mechanism as described in the previous section, there is a homolytic cleavage of the halogen bond due to the energy supplied in the form of heat or light. Let us examine the bond dissociate energies of the halogens, which are shown in Table 14.2.
You will notice that less energy is required to break the Br─Br bond, compared to the Cl─Cl bond. An important consequence of this energy difference is that the bromine radical is more stable and less reactive than the chlorine radical. Let us now look at the next step of the reaction mechanism, the propagation step where the halogen radical abstracts a hydrogen atom from the alkane. You will notice from the dissociation energies given in Tables 14.1 and 14.3 that the energy required to break different C─H bonds varies. More energy is required to break a primary C─H bond, compared to a tertiary C─H bond. This information is presented differently in Table 14.3, which shows the type of radical formed from the cleavage of a different C─H bond.
For alkanes, the tertiary C─H bond is the weakest and hence much easier to break (97 kcal mol−1), compared to the next most difficult, the secondary (99 kcal mol−1) and especially the primary (101 kcal mol−1) and methyl C─H (105 kcal cal−1) bonds. Figure 14.8 gives an illustration of the different types of hydrogens, along with the energy requirements for homolytic cleavage.
Table 14.2 Bond dissociation energies for different halogens.
Bond |
Energy (kcal mol−1) |
F─F |
38 |
Cl─Cl |
58 |
Br─Br |
46 |
I─I |
36 |
Table 14.3 Dissociation bond energies for different C─H bonds.
Hydrocarbon |
Radical |
Type radical |
Bond dissociation energies (kcal mol−1) |
CH3─H |
CH3˙ |
Methyl |
105 |
CH3CH2─H |
CH3CH2˙ |
Primary (1°) |
101 |
(CH3)2CH─H |
(CH3)2CH˙ |
Secondary (2°) |
99 |
(CH3)3C─H |
(CH3)3C˙ |
Tertiary (3°) |
97 |
C6H5CH2─H |
C6H5CH2˙ |
Benzylic |
90 |
CH2═CH─CH2─H |
CH2═CH─CH2˙ |
Allylic |
89 |
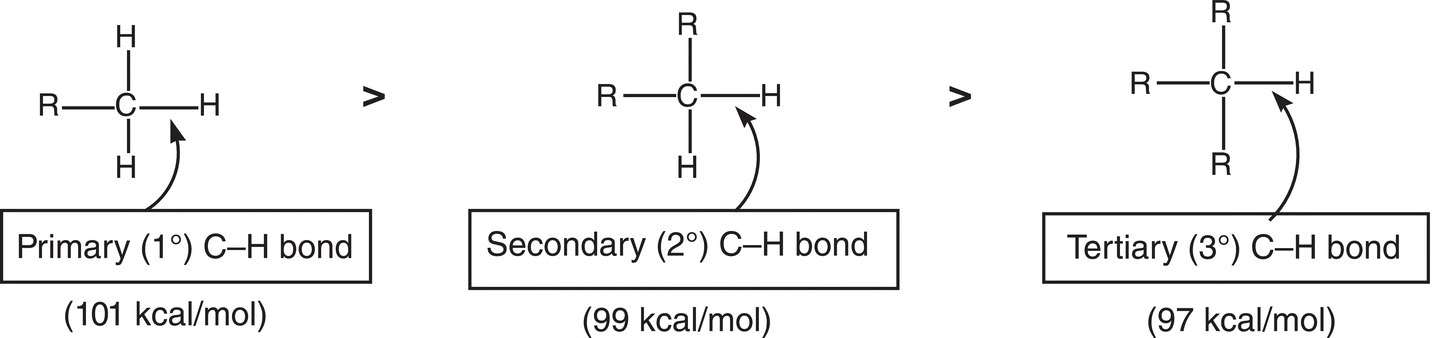
Figure 14.8 Relative strengths of the different types of C─H bonds, the strongest is the primary C─H bond and the weakest is the tertiary C─H bond.
We can utilize the mechanism that was developed to rationalize the different outcomes for these free radical substitution reactions. Let us examine the mechanism for the bromination of propane. The initiation step is shown in Reaction (14-21).
(14-21)
In the next step of the reaction mechanism, the bromine radical, which is fairly stable compared to the chlorine atom, will abstract a hydrogen from propane. For propane, however, there are two types of hydrogens as shown below.
Bromine atom will abstract the hydrogen from the C─H bond that is weakest or will generate a stable secondary radical as shown in Reaction (14-22).
(14-22)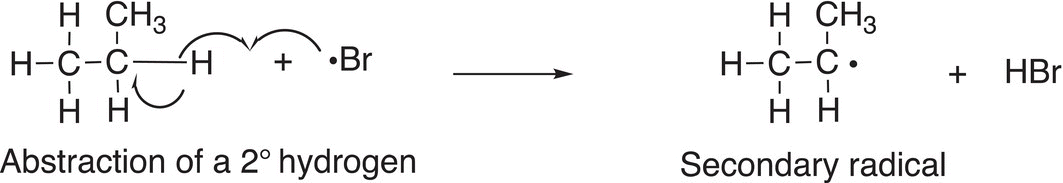
Bromine atom will also abstract the hydrogen from the C─H bond, but with much more difficulty to generate a primary radical as shown in Reaction (14-23).
(14-23)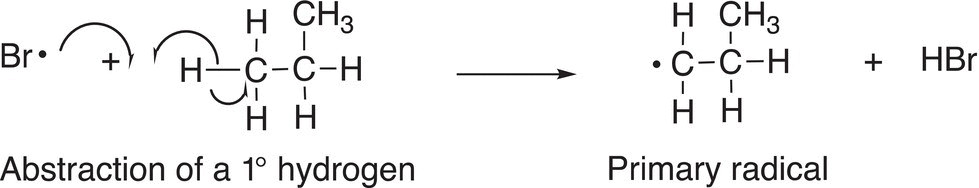
Since the secondary radical is more stable, compared to the primary radical, the major product will result from the addition of bromine radical in a termination step to form the major product, as shown in 14-24.
(14-24)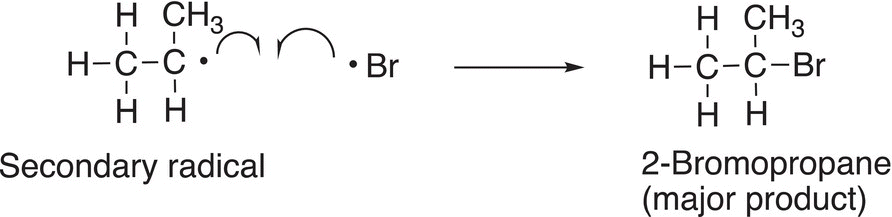
On the other hand, the less stable primary radical will couple with a bromine atom to form the minor product, as shown in Reaction (14-25).
(14-25)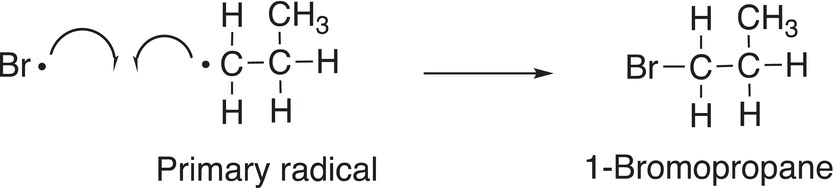
Thus, for the substitution reaction of bromine with propane, the major substitution product will result from the abstraction of the secondary C─H bond, compared to abstraction of the other stronger primary C─H bonds.
With this knowledge, you are now able to predict the major substitution bromination reaction product involving just about any hydrocarbon. You will first have to analyze the different C─H bonds of the starting hydrocarbon. An analysis of 2-methylpropane reveals that it has a tertiary carbon—hydrogen, and a tertiary C─H bond is weaker than either a secondary or primary C─H bond. Thus, the major organic product for the reaction of 2-methylpropane with bromine in the presence of heat or light is 2-bromo-2-methylpropane, and the minor product is 1-bromo-2-methylpropane as shown in Reaction (14-26).
(14-26)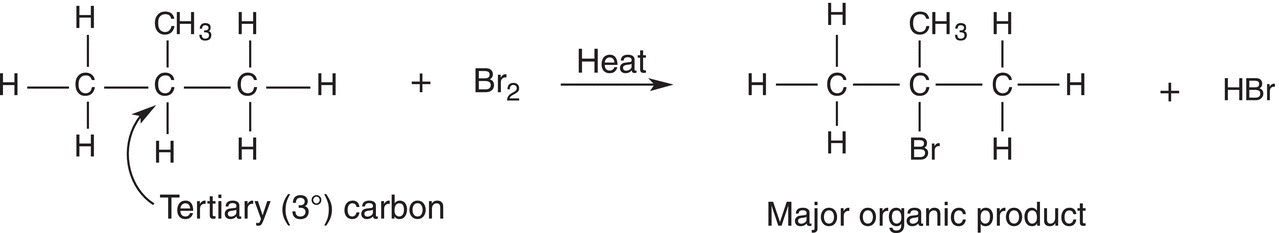
On the other hand, the chlorination of 2-methylpropane will give a mixture of substitution products. As a result, bromine is more selective in a substitution reaction for the weaker C─H bond, compared to chlorine for the halogenation of hydrocarbons.
Problem 14.5
i. Predict the major organic product for the reaction of methylcyclohexane with bromine in the presence of heat (hint: find the weakest C─H bond, i.e. the tertiary C─H bond and that will be the point of substitution).
ii. Complete the following reactions by giving the major organic products.
iii. Predict the major organic products for the bromination of each of the following molecules.
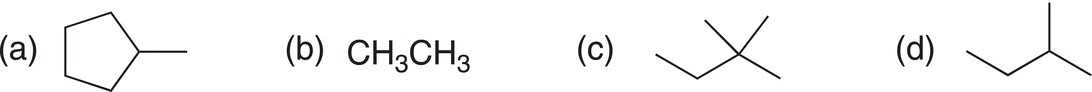
Based on the results of various experiments, it was observed that the bromination of benzylic and allylic compounds occurs almost exclusively at one position, the benzylic or allylic positions, as shown in Reactions (14-27) and (14-28).
(14-27)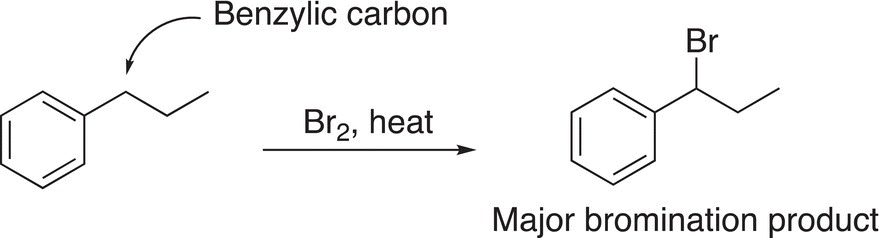
(14-28)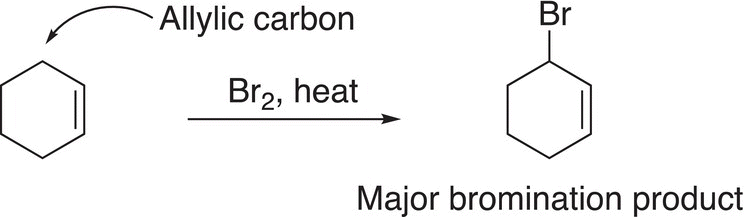
As shown in Table 14.3, the energy required for homolytic cleavage of the benzylic and allylic C─H bonds are 90 and 89 kcal mol−1, respectively. As a result, these bonds are weaker than those of a tertiary or secondary C─H bond, and hence the benzylic and allylic substitution products will be the major products as shown in Reactions (14-27) and (14-28), respectively. At this point, you may be wondering what about the other halogens? The fluorination of methane is an explosive reaction, whereas iodination of alkanes is unfavorable since the iodine radical is extremely stable and hence unreactive.
Problem 14.6
Give a step-by-step mechanism to explain the unexpected product formed for the reaction shown below. That is, give the initiation, propagation, and termination steps for the reaction. Label the different steps of your mechanism.
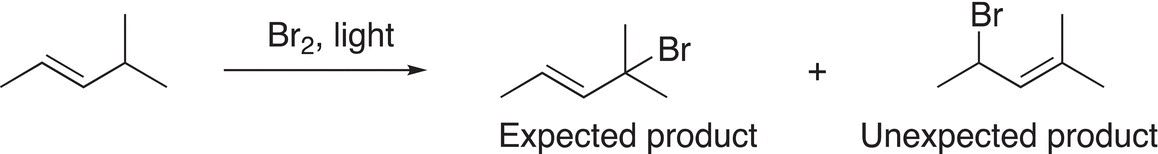
Allylic radicals are readily brominated using a compound called N-bromosuccinamide (NBS), as shown in Reaction (14-29).
(14-29)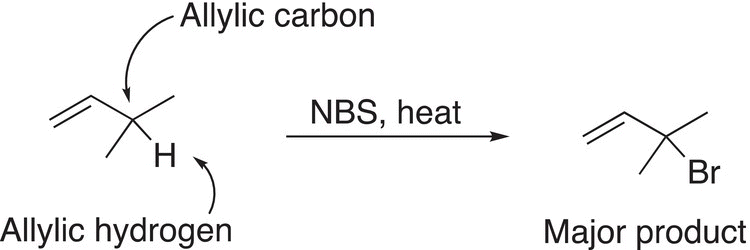
Note that by using NBS, bromine substitution takes place at the allyl position and there is not an addition reaction across the double bond as you might expect since bromine molecule is produced in this reaction. NBS provides a low concentration of bromine so that substitution takes place to produce the more stable allylic radical and bromine does not add across the double bond. In the first step of the reaction using NBS, the N─Br bond, which is a weak bond, is broken in the presence of heat or light, as shown in Reaction (14-30).
(14-30)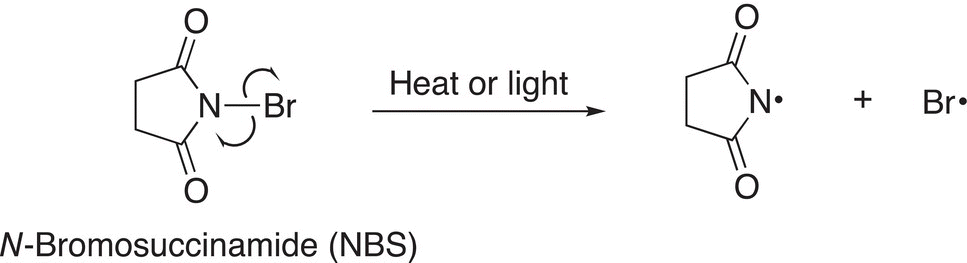
Once the bromine radicals are produced, it abstracts a hydrogen atom from the allylic position of the reactant to produce the stable allylic radical, as shown in Reaction (14-31).
(14-31)
Since this reaction also generates a very strong acid, HBr, it reacts with more NBS as shown in Reaction (14-32).
(14-32)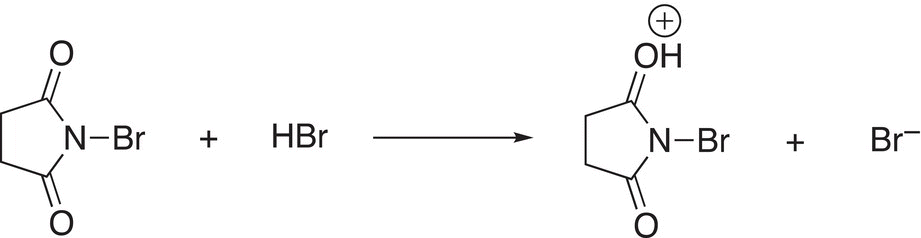
This next step involves a substitution reaction of the bromide anion to form bromine as shown in Reaction (14-33) to form bromine and succinamide anion.
(14-33)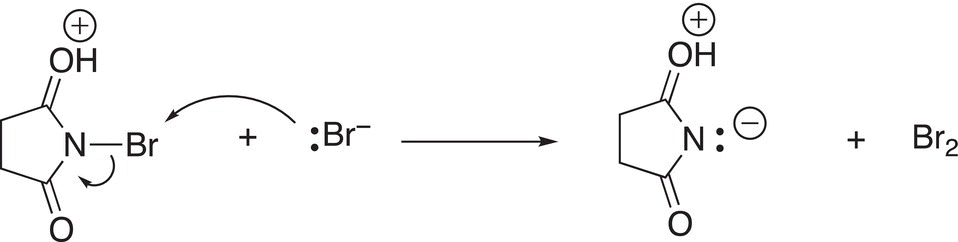
The next step of this reaction involves a proton transfer to generate succinamide as shown in Reaction (14-34).
(14-34)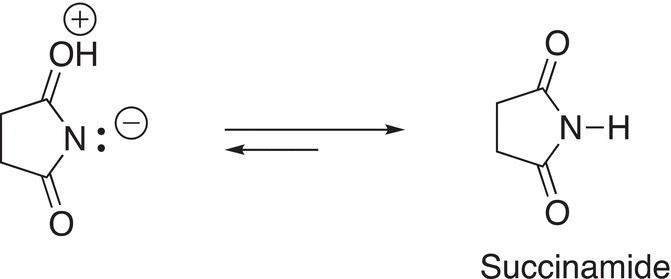
Once there is a constant and low concentration of bromine as generated as shown in Reaction (14-33), it will react as we have seen in the previous sections to give the brominated substitution product and another bromine radical.
(14-35)
In the last steps of the reaction, radicals react to form neutral molecules as shown in Reactions (14-36) and (14-37).
(14-36)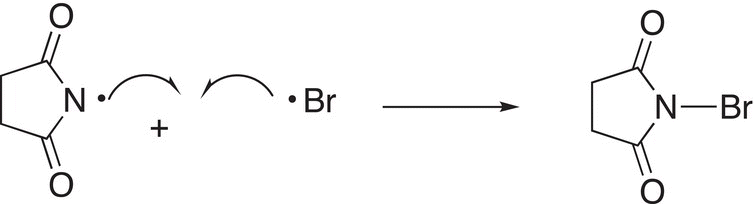
(14-37)
Problem 14.7
Predict the major organic product for the bromination of each of the following molecules in the presence of NBS and heat.
A consequence of a reaction that proceeds through a mechanism that has a radical as an intermediate is that racemic products are produced if there is a chiral carbon in the product. Since the intermediate radical is planar, the addition of the bromine radical in the termination step will take place equally from opposite sides of the radical resulting in a racemic mixture for molecules that have a chiral center at the point of substitution as shown in Reaction (14-38).
(14-38)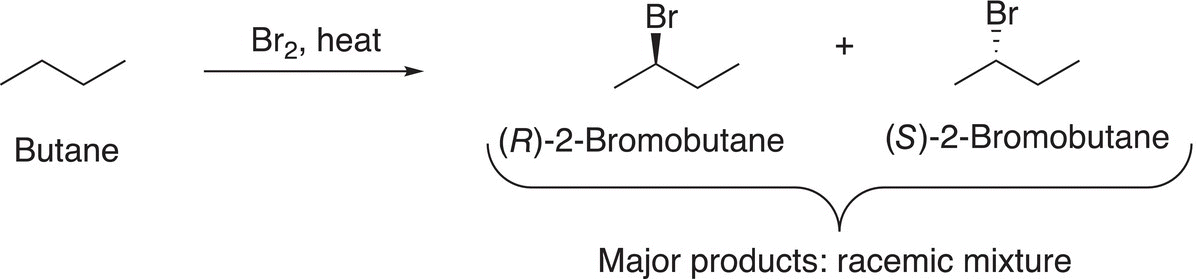
The same is true for a reaction in which the reactant is chiral, the stereochemistry is lost in the product, as shown below in Reaction (14-39).
(14-39)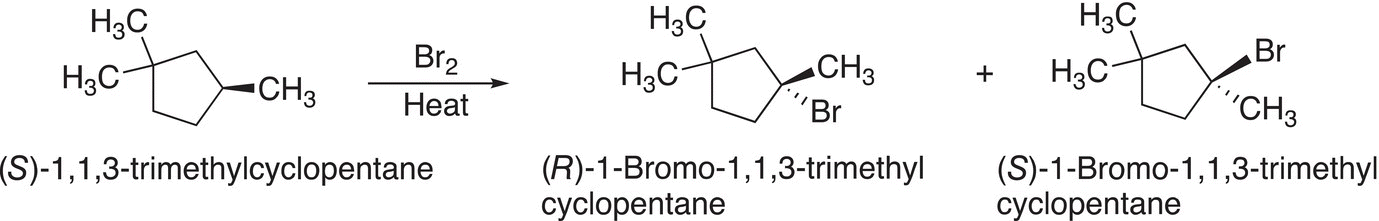
For the halogenation of alkanes, bromine is more selective and there is regioselectivity for these reactions. For the bromination of compounds that have allylic or benzylic hydrogens, the use of NBS serves to maintain a low concentration of bromine and bromine radicals to reduce bromination of the double bond and other reactions that will occur in the presence of a high concentration of bromine.