Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Free Radical Substitution Reactions Involving Alkanes
14.7 Environmental Impact of Organohalides and Free Radicals
Ozone is very important to our existence in that it protects us from the harmful radiation of the sun. It exists in equilibrium with oxygen and oxygen radical as shown below. This means that ozone is constantly produced and destroyed in the atmosphere.
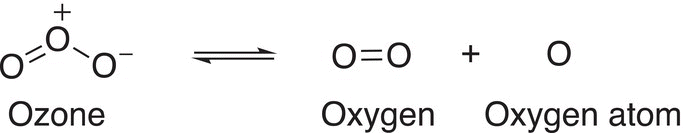
This delicate equilibrium is very important in that the forward reaction is initiated by light as shown below.
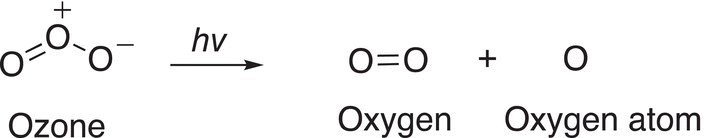
The reverse is spontaneous and produces heat as shown below.

A major benefit of this process is that ultraviolet light from the sun is essentially converted to heat through this process. A major problem encountered in the use of organohalides is that they eventually deplete the ozone in the atmosphere. Organic halides have been used as refrigerants for air conditioners and refrigerators. They have been used also as propellants for various sprays. Some of the commonly used ones are shown below.
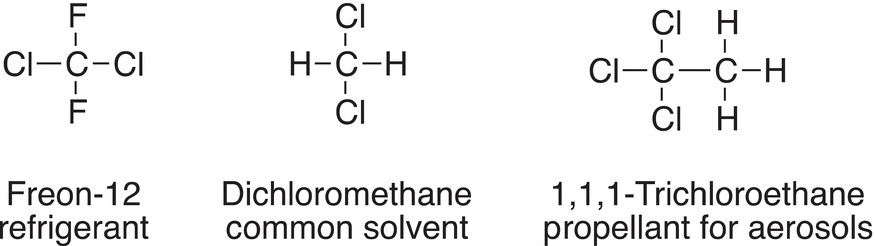
Several metric tons of these organohalides, also called CFCs, are released into the atmosphere yearly. Unfortunately, these compounds pose various challenges for the environment. For example, Freon-12, in the presence of light, breaks apart to give chlorine atoms, as shown below.
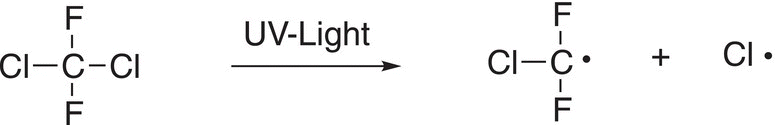
There is a reaction between chlorine atoms and ozone as shown below.

As you can see in this propagation step, more radicals are produced, in addition to oxygen, but most important, ozone has been consumed in this process. There are many possible propagation steps, which eventually generate more chlorine atoms as shown below.
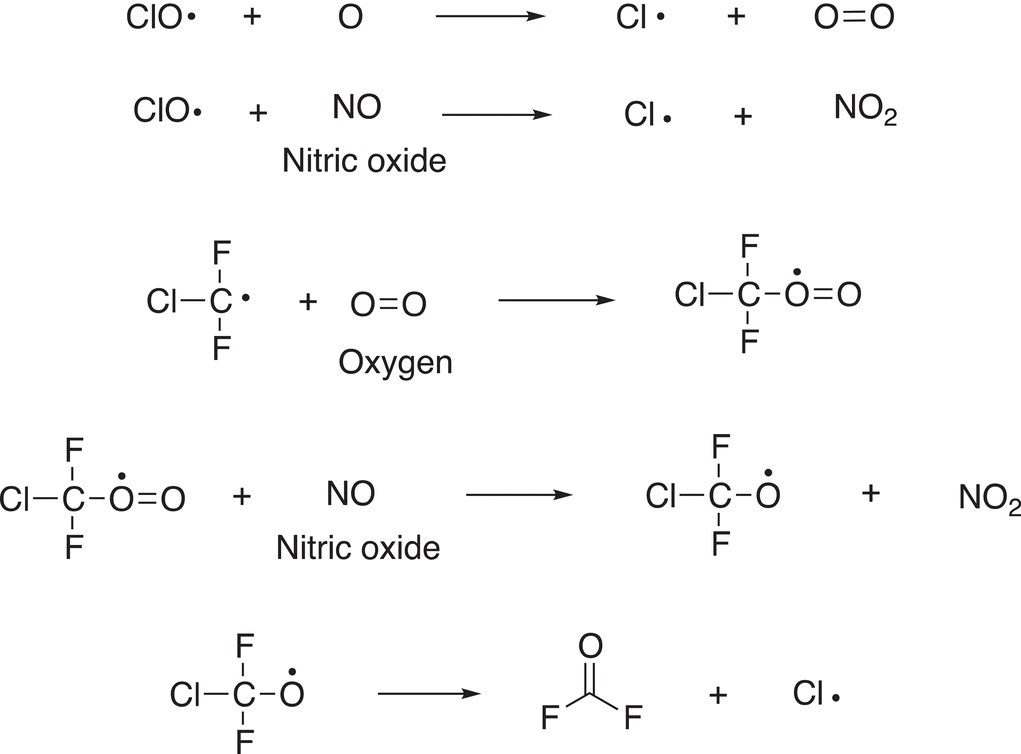
These are all propagation steps that generate chlorine radicals, and chlorine radicals are very destructive to the ozone layer.
Hydroflurorcarbons (HFCs) were used to replace CFC. These compounds were thought to be better than CFC since they contain a weaker C─H bond, compared to a C─Cl bond and, as a result, are more easily destroyed in the atmosphere, where hydroxide radicals are present, and these HFCs could be destroyed before they reach the stratosphere where most of the ozone exists. The rational is that these HFCs do not generate the destructive chlorine radicals.
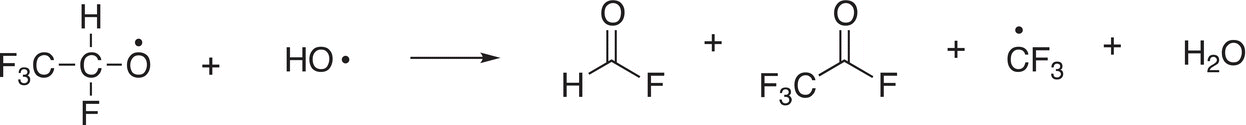
Some of the propagation steps in which the hydroxide radicals are used are shown below.
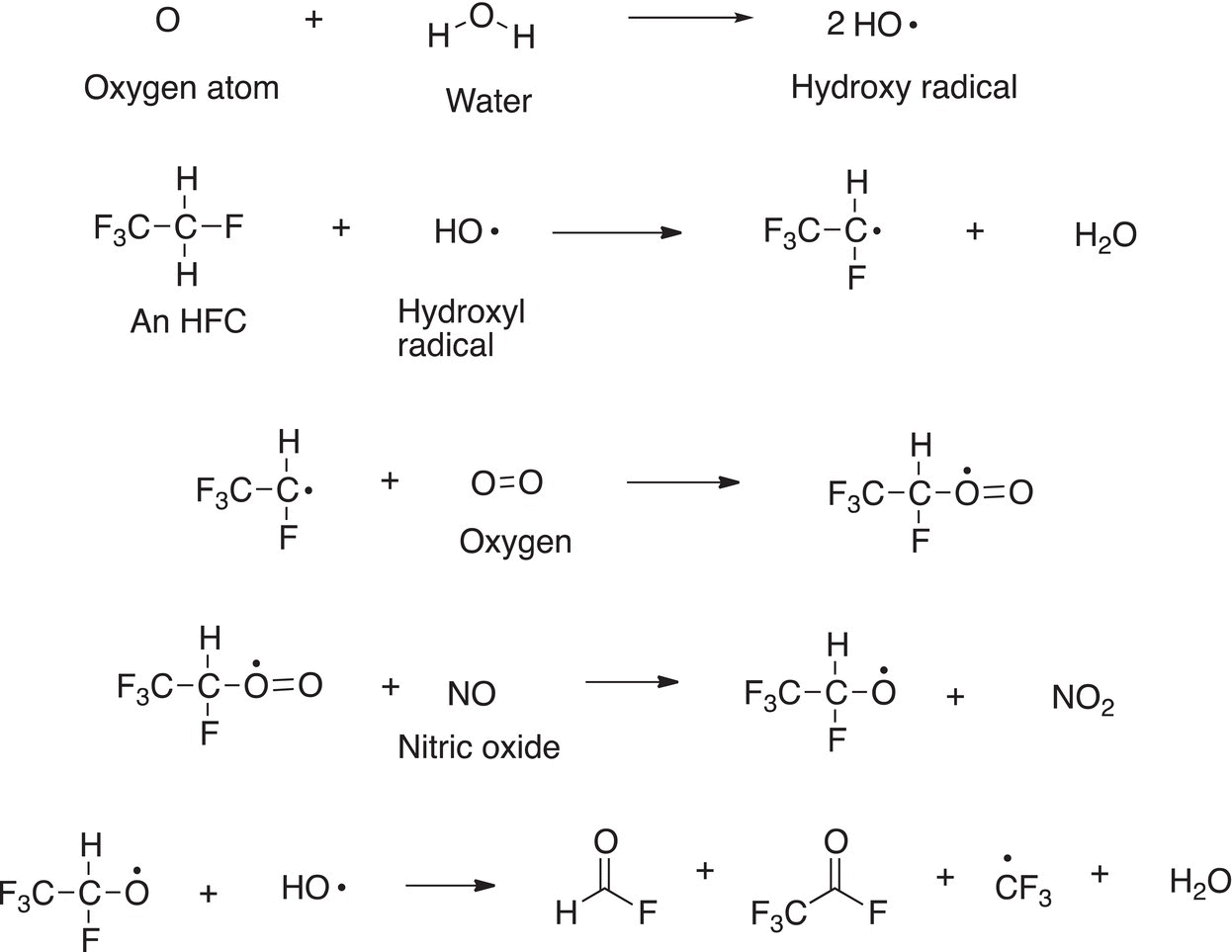
Before too long after the introduction of these compounds, they came under much scrutiny, and recently, negotiators from more than 170 countries reached a legally binding accord in Kigali, Rwanda, to drastically reduce the use of HFCs.
Problem 14.10
The propagation step of a free radical reaction is shown below. By using the curved arrow formulism, show how all the products given are formed.