Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at sp3 Carbons
15.5 Nucleophilic Substitution Reactions
As the name suggest and as mentioned in Chapter 6, the nucleophilic substitution reaction involves a nucleophile reacting with an electrophile in which the leaving group in the reactant is substituted by the nucleophile to form a product. The general feature of a nucleophilic substitution reaction is shown in Reaction (15-10).
(15-10)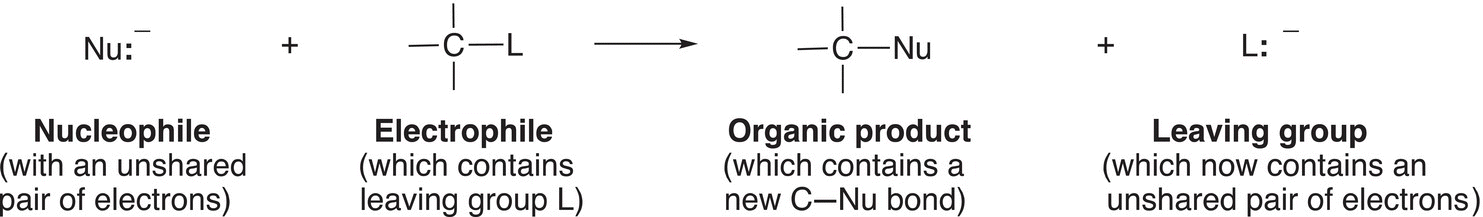
An example of a nucleophilic substitution reaction is shown in Reaction (15-11), which involves the reaction of chloroethane and hydroxide anion.
(15-11)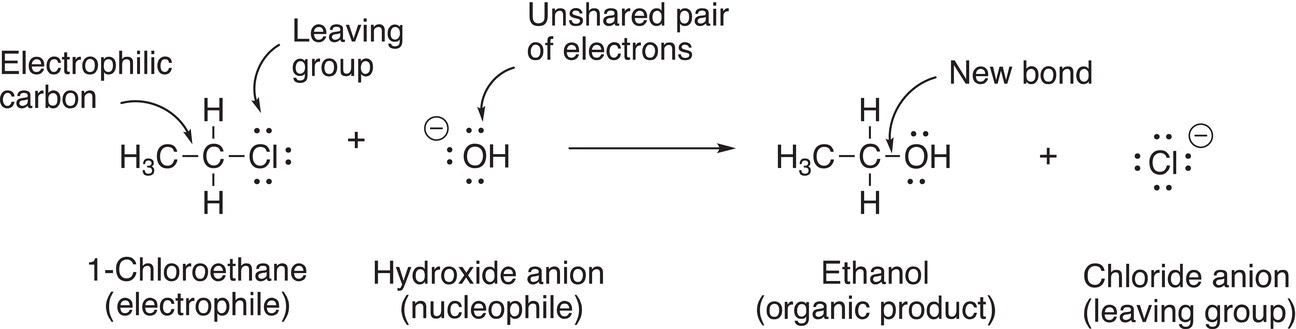
You should be able to identify some important species of Reaction (15-11); the first is the electrophile in the reaction. After you have identified the electrophile, you should be able to identify the electrophilic atom. Next, you should be able to identify the nucleophile. Once the nucleophile has been identified, you should be able to identify the nucleophilic atom and the unshared pair of electrons that are used to form the new bond in the product. The next is the leaving group that is typically bonded to the electrophilic atom of the electrophile, in this case, chlorine is the leaving group since it is substituted for an OH in the product and it is a stable anion (weak base). You should recognize that a major driving force for these substitution reactions to occur is that owing to the partial positive character of the electrophilic carbon of electrophiles, an attraction of the unshared pair of negative electrons and the nucleophile occurs and in the process the leaving group leaves. Thus, a reaction between a nucleophile and an electrophile is favorable because these species have opposite charges (or partial charges) and opposite charges (or partial charges) always attract each other. In the process of the reaction, the leaving group leaves because it can accommodate a formal negative charge (or a neutral molecule in some cases, as we have seen with H2O and R3N). Shown in Reactions 15-12 and 15-13 are examples of substitution reactions with different leaving groups and nucleophiles.
(15-12)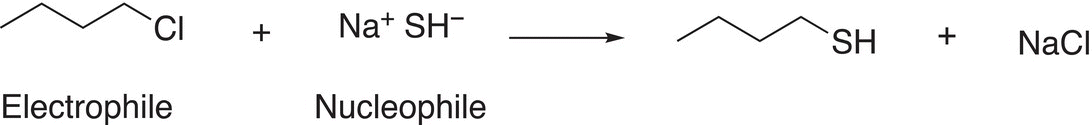
(15-13)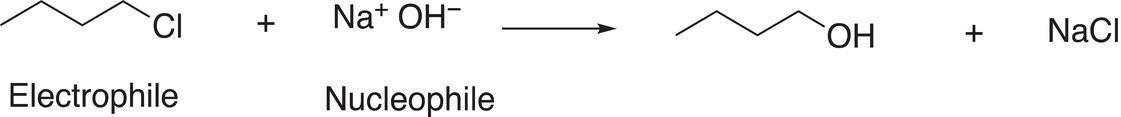
The leaving group can give information about a substitution reaction; a reaction that contains an electrophile with a good leaving group is a better electrophile for a substitution reaction, compared to another reaction in which the electrophile has a poor leaving group. Consider the reactions shown in Reactions (15-14) and (15-15). For Reaction 15-14, the leaving group is the chloride anion, which is not as good a leaving group as the iodide anion shown in Reaction (15-15).
(15-14)![]()
(15-15)![]()
Note that if the nucleophile is a salt, the cation is ionically bonded to the leaving group to form a salt in the product as shown in Reaction (15-16).
(15-16)
On the other hand, if the nucleophile is bonded to a proton, such as H2O or NH3, the proton is bonded to the leaving group in the product, as shown in Reaction 15-17.
(15-17)
Problem 15.4
Which of the two reactions shown below is the most favorable substitution reaction? Explain your answer.
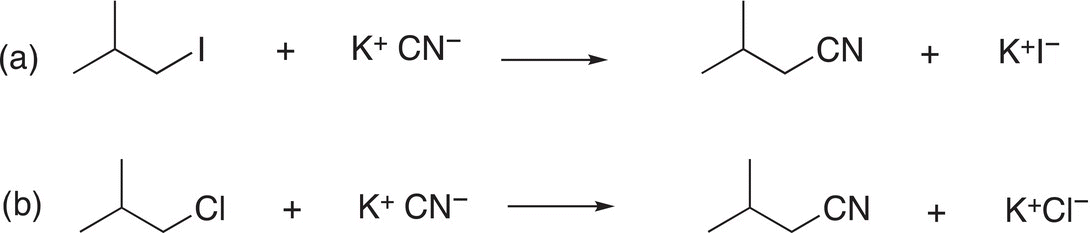
Once the electrophile, nucleophile, and leaving group have been identified, it is easy to predict the products of substitution reactions, and Problem 15.5 is designed to assist students recognize these features of substitution reactions and to apply that knowledge to predict the products of substitution reactions.
Problem 15.5
Give the products (both organic and inorganic) for the following reactions. Hint: be sure to identify the electrophilic and nucleophilic atoms before predicting the products.
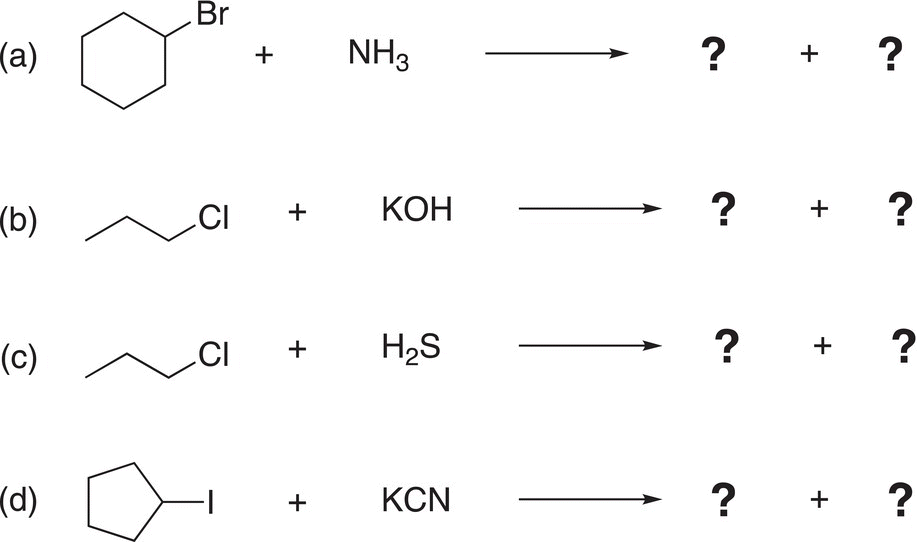
15.5.1 Mechanisms of Nucleophilic Substitution Reactions
In this section, we will examine possible routes for substitution reactions. One possibility is that the nucleophile attacks the electrophile from the rear and pushes out the leaving group and in the process a new carbon—nucleophile bond is formed. Another possibility by which the reaction can take place is for the leaving group to depart first, without the assistance of the nucleophile, to form a fully positive species, also known as a carbocation, and the leaving group. In a second separate step, the nucleophile attacks the positive carbocation to form a carbon—nucleophile bond of the product. Note that either of these two routes gives the same substitution products. These different possible routes are referred to as mechanisms and they have specific names, bimolecular nucleophilic substitution (SN2) is the term that is used to describe the first mechanism; and unimolecular nucleophilic substitution (SN1) is the term used to describe the second mechanism. In the next sections, we will go into these mechanisms in more detail.