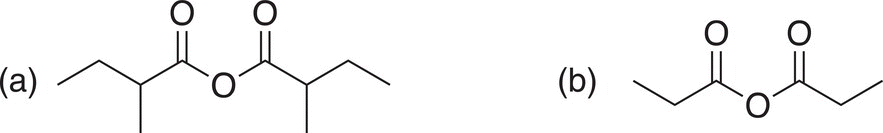Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at Acyl Carbons
16.4 Substitution Reactions Involving Anhydrides
There are two important features of anhydrides that lead to the type reactions observed for these molecules. First, the carbonyl double bond is a polarized bond. Thus, the carbon will accept a nucleophile. Second, anhydrides have a very good leaving group in the form of the carboxylate anion. Thus, similar substitution reactions will take place at the carbonyl carbon of anhydrides of the reactions of acid chlorides. A major difference, however, between the reactions of acid chlorides and anhydrides is that the leaving group for anhydrides is not as good a leaving group as the chloride anion. You will recall from Chapter 7 on acids and bases that the pKa of carboxylic acids is approximately 4.7, whereas the pKa for HCl is −7.0. This difference in pKa values indicates that the chloride is a much better leaving group, compared to the carboxylate anion. The general reaction of anhydrides with a nucleophile is shown in Reaction (16-28).
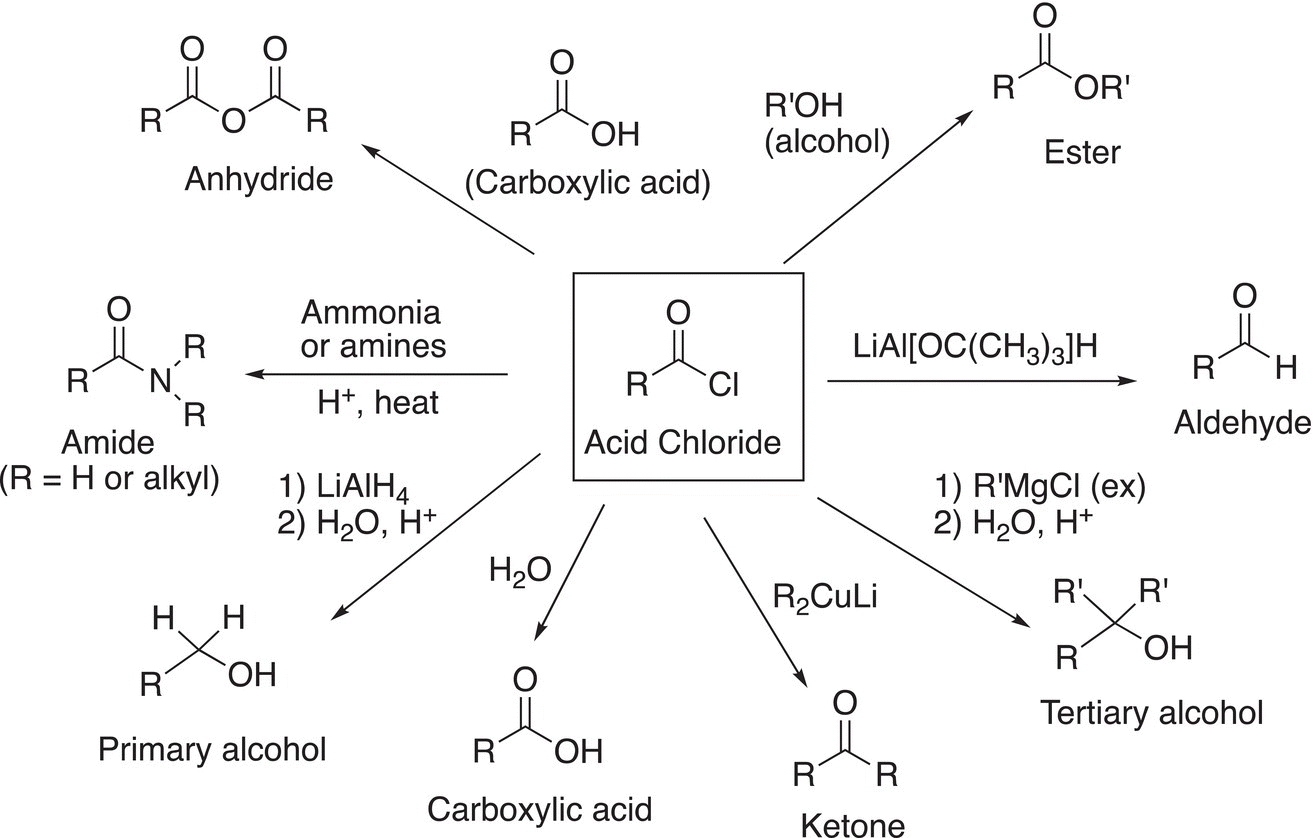
Figure 16.1 Summary of the reactions of acid chlorides.
(16-28)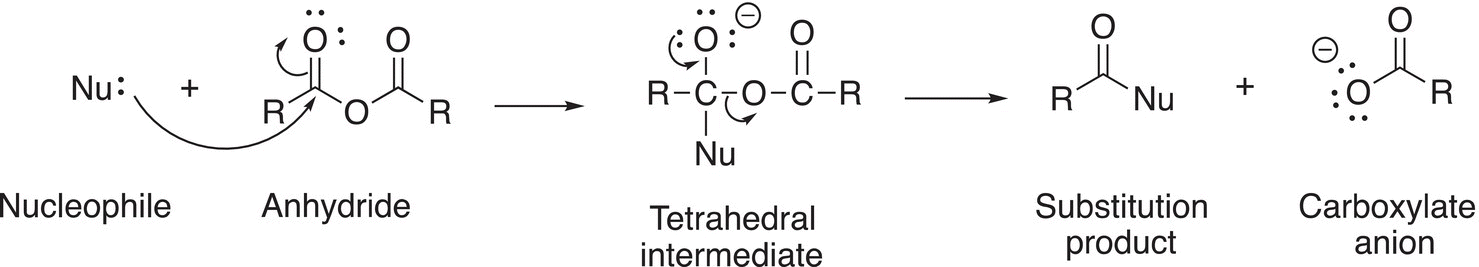
The first step of the reaction mechanism is the addition of the nucleophile to one the carbonyl carbons of the anhydride. The stability of the resulting intermediate determines the overall reactivity of anhydrides. The second step is the elimination of the carboxylate anion, RCOO−. Thus, the overall reaction is a nucleophilic substitution reaction at a carbonyl carbon in which a RCOO− anion is substituted by Nu− to form the product. Note that since both carbonyl carbons of the anhydride are electrophilic, there is a probability that the nucleophile will attack either carbonyl carbon to give a mixture of organic products if the R groups are different. Thus, if this type of reaction is used as a synthetic reagent, the anhydrides should be symmetric anhydrides. Owing to the mixture of organic products that result from the reaction of unsymmetrical anhydrides with a nucleophile, most of the anhydrides that are used in the laboratories are symmetrical anhydrides.
16.4.1 Substitution Reactions of Anhydrides with Water
The reaction of anhydrides with water is very similar to that of the hydrolysis of acid chlorides, except as pointed out earlier the leaving group for the anhydride is the carboxylate anion and in the presence of an acidic medium, another mole of carboxylic acid is formed as shown in Reaction (16-29).
(16-29)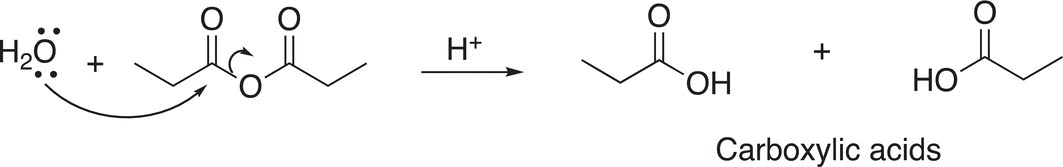
As you can imagine, the size of the alkyl group bonded to the carbonyl carbon dictates the reactivity of anhydrides. Anhydrides with large groups react slower than anhydrides with smaller groups bonded to the carbonyl carbon. Examples of the relative reactivity of two anhydrides are shown below.
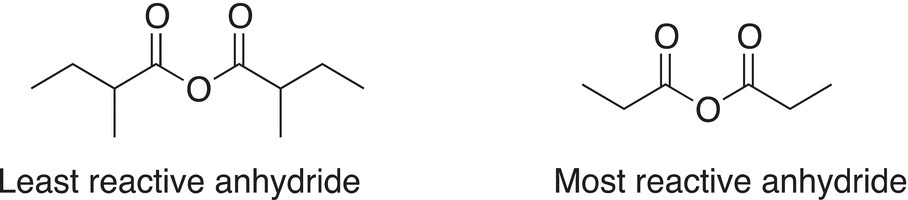
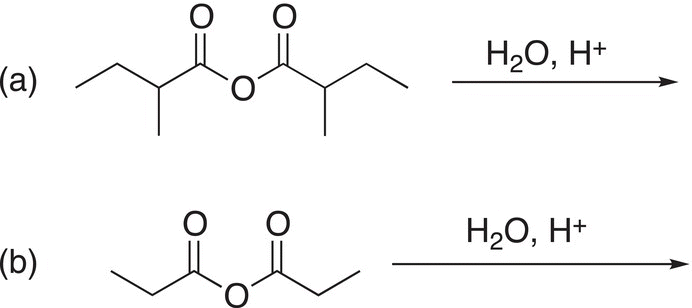
Problem 16.10
Give the major organic products of the following reactions.
16.4.2 Substitution Reactions of Anhydrides with Alcohols
The reaction of acetic anhydride with methanol is shown in Reaction (16-30). You will notice that based on your knowledge of the reactions of acid chlorides with alcohols, the outcome of the reaction of anhydrides with alcohols is not surprising.
(16-30)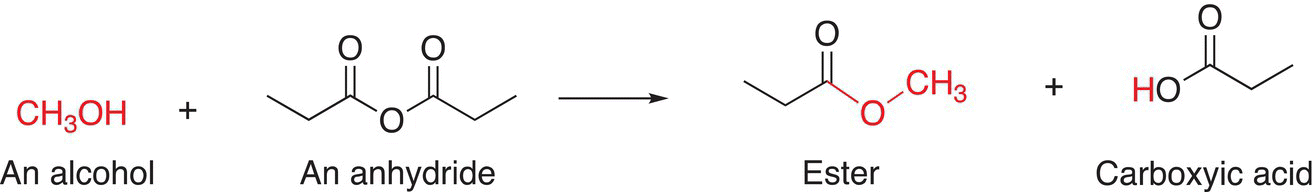
In the first step of the reaction mechanism, the nucleophilic alcohol reacts with the electrophilic carbonyl center to form a tetrahedral intermediate, which is followed by a proton transfer as shown in Reaction (16-31).
(16-31)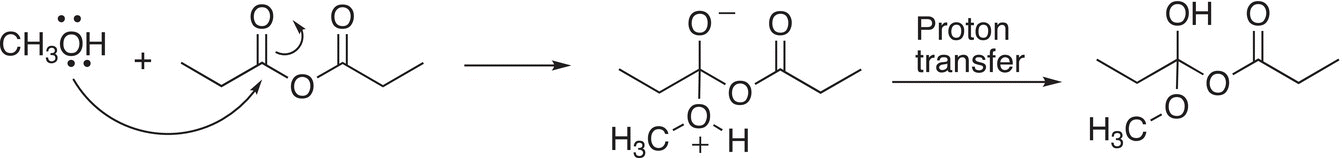
In the next step of the mechanism, the tetrahedral intermediate forms a carbon—oxygen double bond as the leaving carboxylate anion leaves as shown in Reaction (16-32).
(16-32)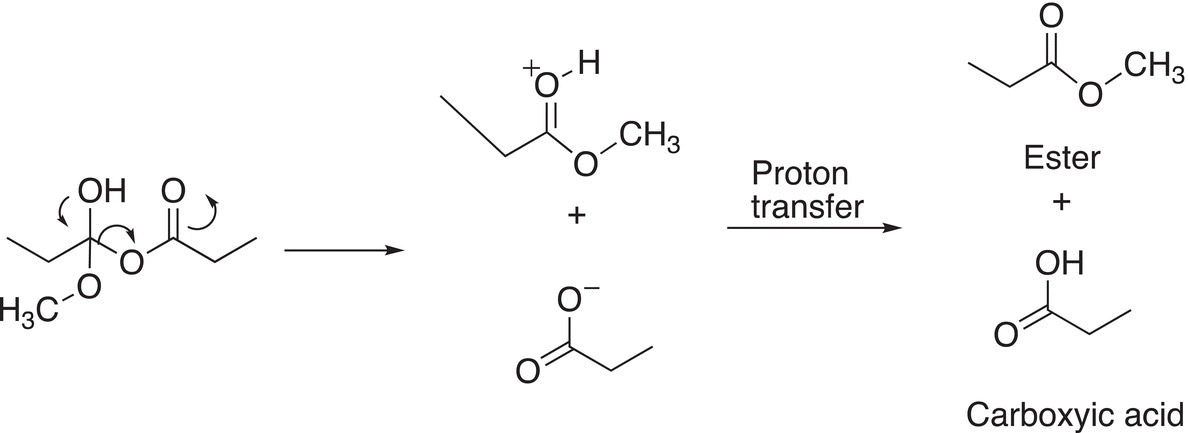
The products of these reactions are esters and carboxylic acids.
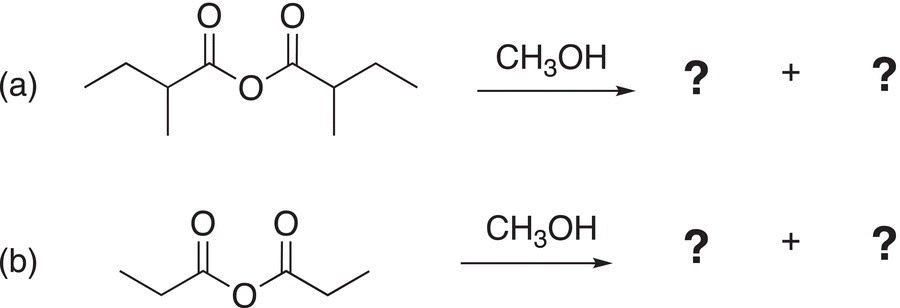
Problem 16.11
Give the products of the following reactions.
16.4.3 Substitution Reactions of Anhydrides with Ammonia and Amines
Since ammonia and amines are nucleophilic molecules, they will react with anhydrides to form primary amides, secondary amides, and tertiary amides, and of course, carboxylic acids. The carboxylic acid that is produced in the reaction, reacts with excess ammonia or amines to form the corresponding ammonium salt. The mechanism for these types of reactions is similar to that shown in the previous section, except that the nucleophile in this case is the nitrogen of ammonia or amine. The first step of the mechanism involves the bonding of the nucleophilic nitrogen to the electrophilic carbonyl carbon to form a tetrahedral intermediate, followed by a proton transfer as shown in Reaction (16-33).
(16-33)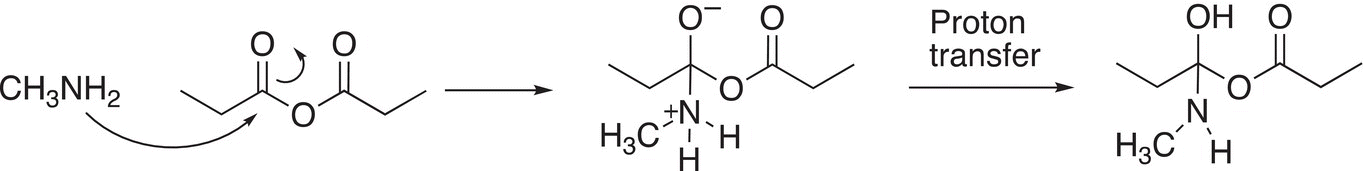
In the next step of the mechanism, the carbon—oxygen double bond forms and the carboxylate anion leaves as shown in Reaction (16-34) to form the amide and carboxylic acid products.
(16-34)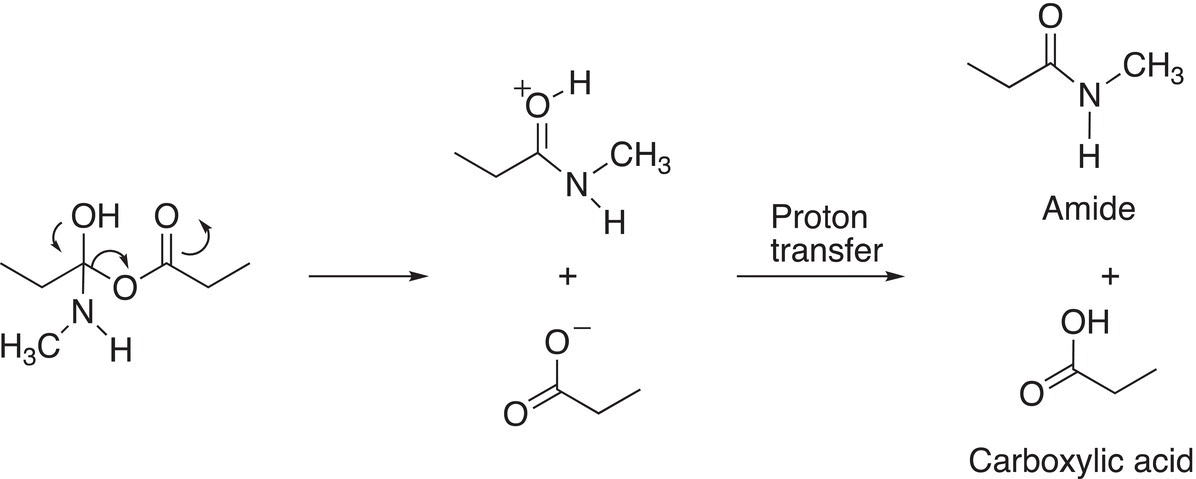
Problem 16.12
Give the products for the reaction of each of the molecules shown below with ethanoic anhydride (ethanoic ethanoic anhydride).
1. CH3NH2
2. NH3
3. (CH3CH2)2NH
4. CH3CH2NH2
16.4.4 Substitution Reactions of Anhydrides with Carboxylate Salts
As you can imagine, the reaction of an anhydride with carboxylate salts gives another anhydride and a carboxylate salt. These reactions are not very useful for the synthesis of new compounds since they generate a nonsymmetric anhydride as shown in in the example given in Reaction (16-35).
(16-35)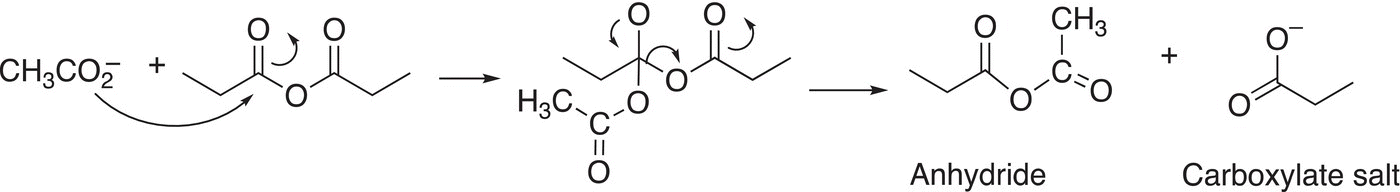
Problem 16.13
Give the product for the reaction of each of the molecules shown below with sodium ethanoate.
16.4.5 Substitution Reactions of Anhydrides with Soft Organometallic Reagents
A soft reducing agent, such as the organocuprate, will react with anhydrides to give the corresponding ketone and carboxylate salt, as shown in Reaction (16-36).
(16-36)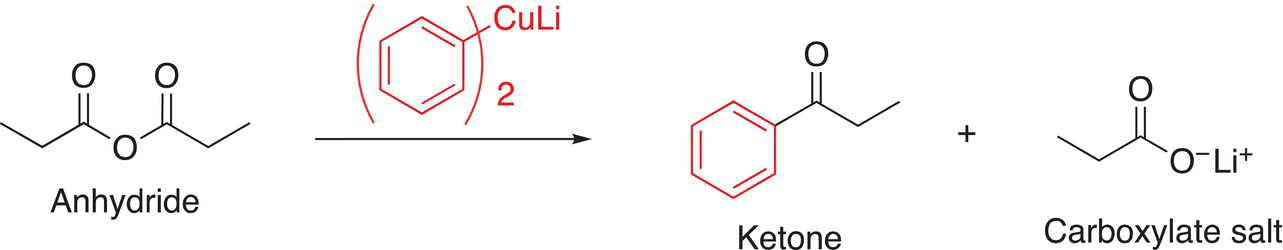
Problem 16.14
Give the products for the reaction of each of the molecules shown below with diethyl lithium cupurate.

16.4.6 Substitution Reactions of Anhydrides with Hard Organometallic Reagents
As we have pointed out earlier in Chapter 10 when we examined reducing agents, organometallic reagents in addition to being very strong bases and good reducing agents, they are also good nucleophiles. Grignard reagents, for example, react with anhydrides to produce tertiary alcohols as the final product after hydrolysis, as shown in Reaction (16-37).
(16-37)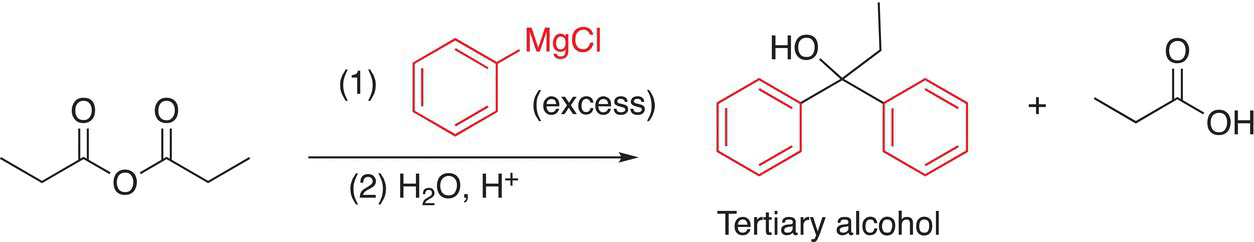
Problem 16.15
Give the products for the reaction of each of the molecules shown below with phenyl Grignard reagent (C6H5MgCl), followed by acid hydrolysis.

16.4.7 Substitution Reactions of Anhydrides with Soft Metallic Hydrides
The reaction of anhydrides with soft metal hydrides, such as lithium di-tert-butoxide hydride, results in aldehydes and carboxylate salts. As you would expect, the reaction will add just one mole of a hydride ion to produce an aldehyde as a final product, as shown in Reaction (16-38).
(16-38)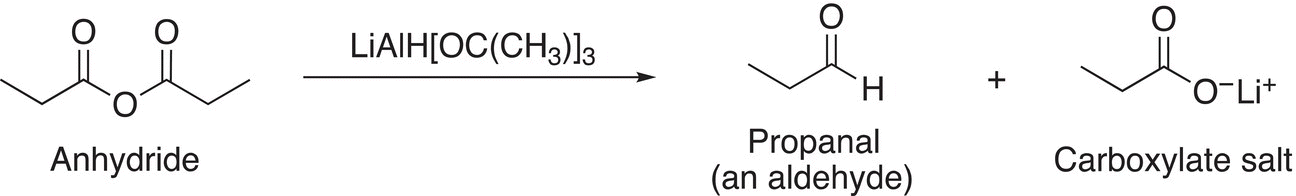
In a second step, the carboxylate salt can be converted to a carboxylic acid by an acid—base reaction.
Problem 16.16
Give the products for the reaction of each of the molecules shown below with lithium tri-tert-butoxyaluminum hydride.
16.4.8 Substitution Reactions of Anhydrides with Hard Metallic Hydrides
On the other hand, the reaction of anhydrides with a much harder nucleophile, in this case, also known as a very strong reducing agent, results in first an acyl substitution as shown in Reaction (16-39).
(16-39)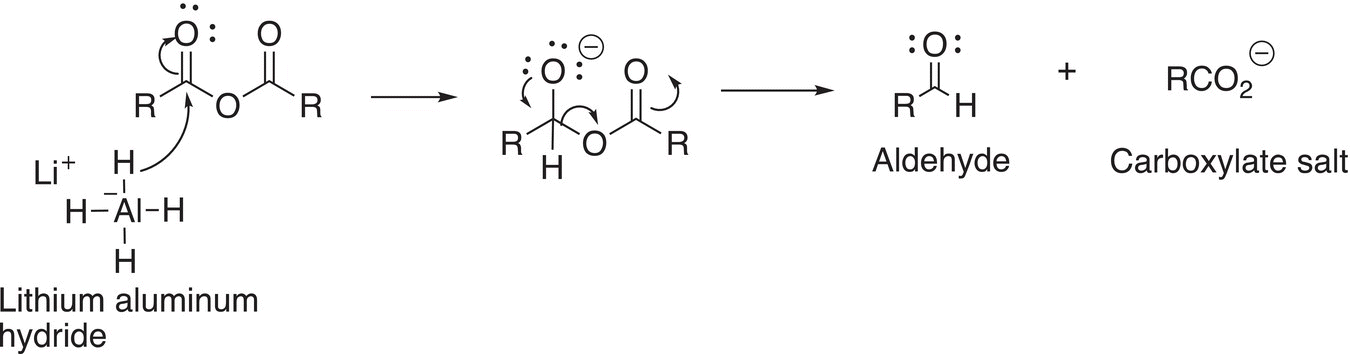
The aldehyde that is produced readily accepts another mole of hydride anion from the reducing LiAlH4 agent to give an alcohol salt, which upon hydrolysis gives a primary alcohol as shown in Reaction (16-40).
(16-40)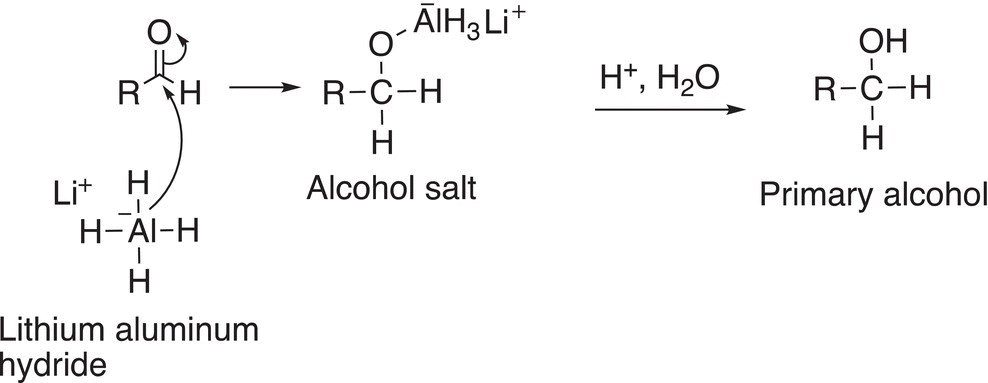
As we will see later in the chapter, the carboxylate salt can be reduced in the presence of a strong reducing agent, such as LiAlH4, to give the corresponding alcohol, as shown in Reaction (16-41).
(16-41)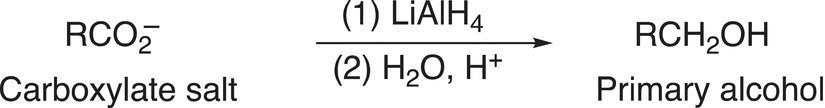
Thus, the final product of a reduction of an anhydride using a strong metal hydride reducing agent, such as LiAlH4, is shown in the examples are shown in Reactions (16-42) and (16-43).
(16-42)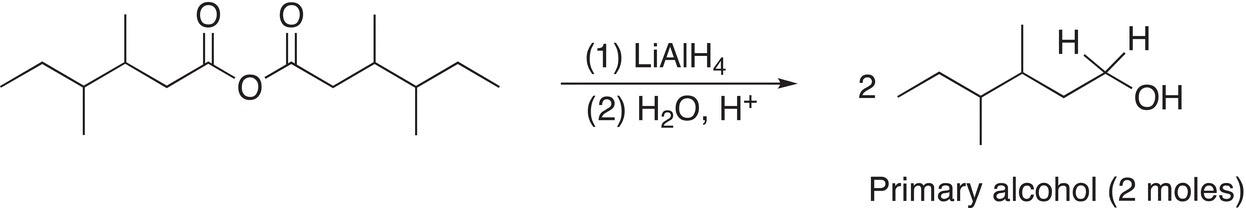
(16-43)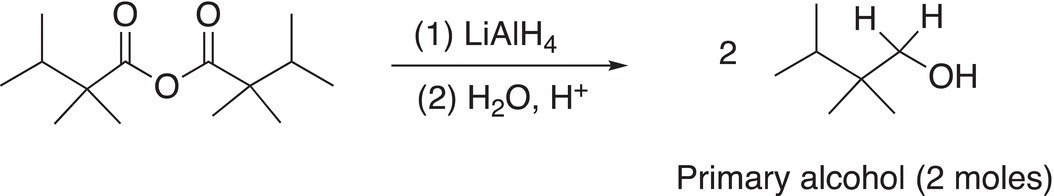
Note that these examples use symmetrical anhydrides so that there are no mixed organic products produced, Reactions (16-44) and (16-45) give examples using unsymmetrical anhydrides.
(16-44)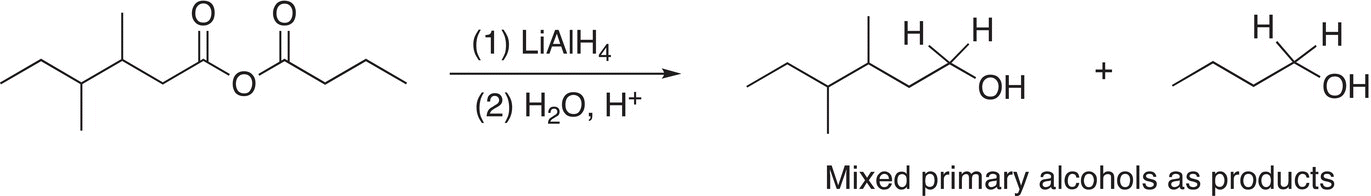
(16-45)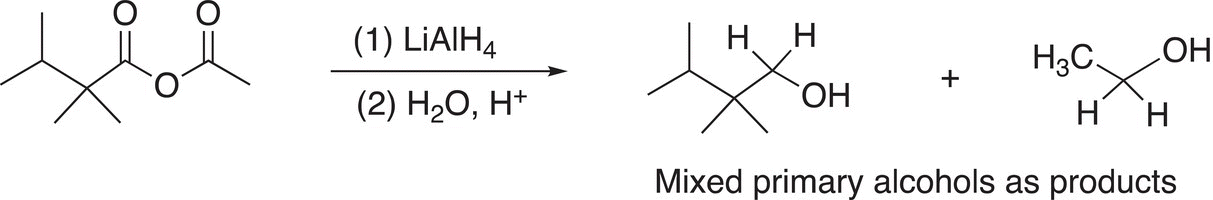
Problem 16.17
Give the organic products for the reaction of each of the molecules shown below with LiAlH4, followed by acidic work-up.