Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Nucleophilic Substitution Reactions at Acyl Carbons
16.10 Applications of Acyl Substitution Reactions
16.10.1 Preparation of Esters
As pointed out earlier, for the synthesis of esters, there are two important bonds that can be made to put this type of molecule together. A bond that can be made is the bond to the carbon of the carbonyl carbon—oxygen bond. This bond can be made from a reaction of the following type. Below is shown a specific example to synthesize propyl ethanoate. The leaving group can of course be a chloride anion, in that case, the starting material is an acid chloride.
(16-111)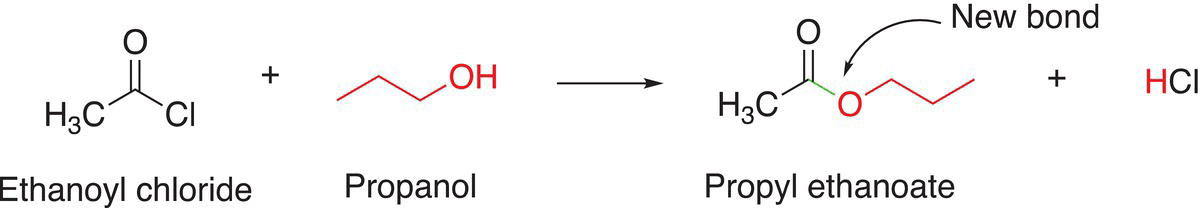
Another molecule that can be used as a starting material is a carboxylic acid. Since the OH is a very poor leaving group, however, such reactions are carried out in an acidic medium to convert the OH into a much better leaving group, OH2. If you were not given the starting two reactants to synthesize the product, propyl ethanoate, would you be able to think of the two reactants needed to synthesize that molecule? The key is to think of how best to make the bond highlighted. So, let us think backward in terms of identifying what is needed to make a new molecule, and specifically the two fragments needed to make a new bond.
(16-112)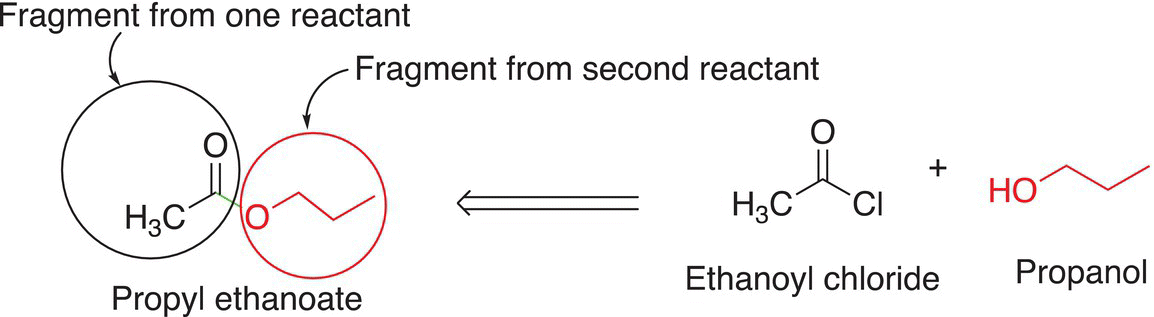
The other method to synthesize the same molecule is shown in Reaction (16-113).
(16-113)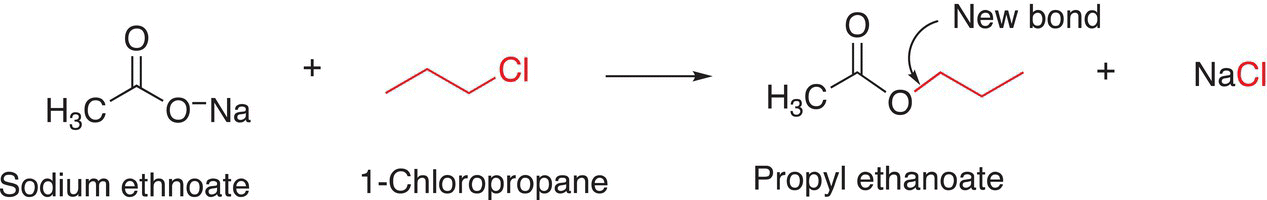
Using the same approach as above, once the bond to be made is recognized, the fragments needed as reactants can be identified as shown above. Note that this reaction involves using sodium acetate as the nucleophile for an SN2 reaction to react with a primary halide to create the target molecule.
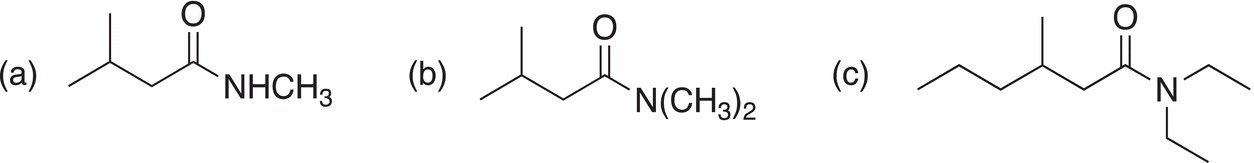
Problem 16.29
Using the method outlined above, try to figure out two synthetic routes for the synthesis of the molecules shown below.
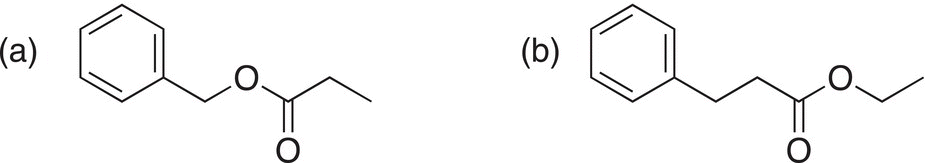
16.10.2 Preparations of Amides
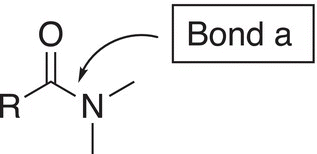
For the synthesis of amides, there is one important bond that should be considered. This bond is the carbonyl carbon—nitrogen bond. This bond can be made from a substitution reaction at a acyl carbon. Amides can be made by synthesizing the carbonyl carbon—nitrogen bond, bond a shown below.
We have seen this type of reaction before when we used ammonia or amines to react using a substitution at a carbonyl carbon as shown in Reaction (16-114).
(16-114)
Since acid chlorides are the most reactive molecules by a substitution reaction at an acyl carbon, they are frequently used to make the carbonyl carbon—nitrogen bond of the amide as shown in Reaction (16-115).
(16-115)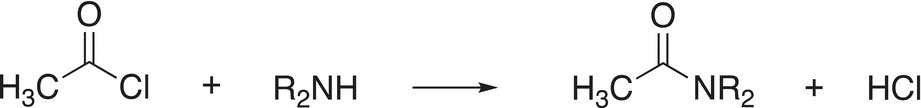
Cl is used as a good leaving group, but any other good leaving group could be used. Groups such as the carboxylate anion (anhydrides) or alkoxide (esters) can be used.
Consider the synthesis of the N,N-dimethylacetamide, shown below.
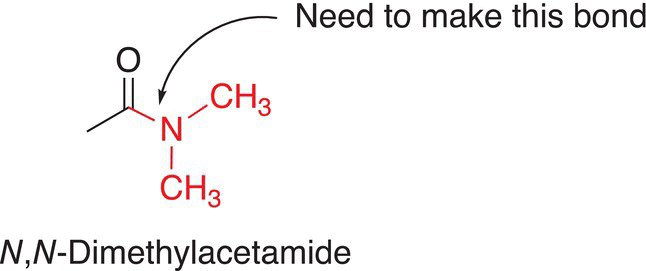
Two strategies are shown below, in which an electrophilic anhydride and nucleophilic dimethyl amine are used as starting reagents as illustrated in the reaction scheme given in Reaction (16-116).
(16-116)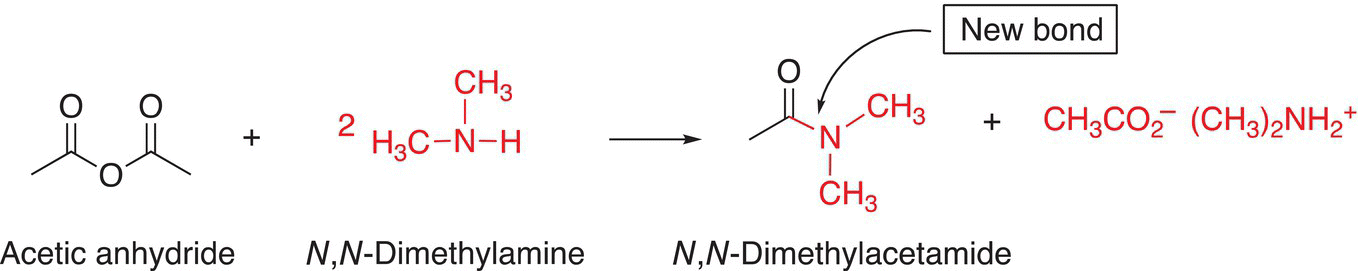
It is possible to use a different electrophile and same nucleophile to synthesize the target molecule, as shown in Reaction (16-117)
(16-117)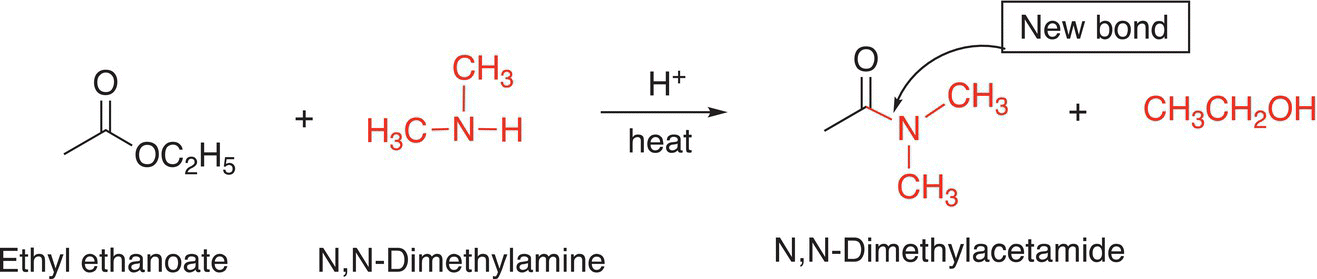
Problem 16.30
Give appropriate starting reagents that could be used for the synthesis of the amides shown below.