Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Heteroatomic Functional Groups and Organic Nomenclature
3.9 Structure and Properties of Esters
Esters have the functional group as shown below, in which an alkyl group is bonded to the carbon of the carbon—oxygen double bond and another alkyl group is bonded to oxygen as shown below.

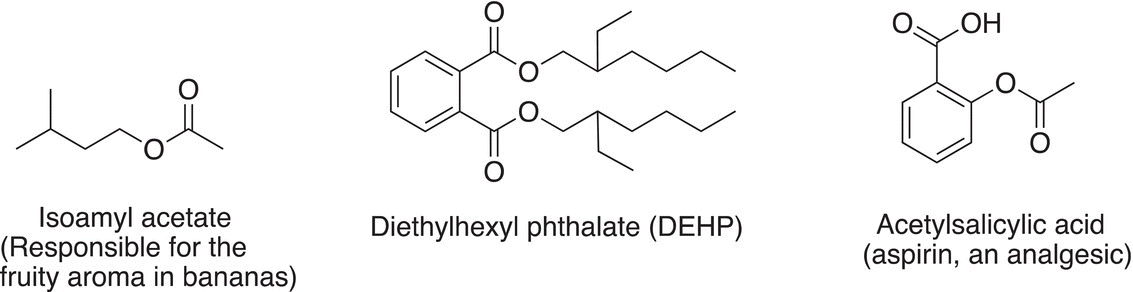
Molecules that contain the ester functionality are also known as esters and they are typically sweet-smelling compounds, and the sweet aroma of most fruits is due to the presence of esters. For example, isoamyl acetate is the ester responsible for the banana aroma of ripened bananas.
DID YOU KNOW?

An ester, known as isoamyl acetate, is responsible for the sweet-smelling aroma of ripened bananas.
Esters are also good solvents and can even dissolve body tissue. Diethylhexyl phthalate (DEHP), which is an ester, is used as plasticizer to impart flexibility to polyvinyl chloride (PVC) plastics. Aspirin (acetylsalicilic acid), which was mentioned earlier as an analgesic, also contains an ester functionality.
Problem 3.15
For the molecules shown above, identify and circle the ester functional group.
3.9.1 Nomenclature of Esters
In naming esters, first identify the ester functionality and name the group bonded to the oxygen of the ester group as an alkyl group. The group that is bonded to the carbon of the carbonyl group is named as an alkanoate; the format of the combined IUPAC name is shown below. Note that there are two words in the name of esters.
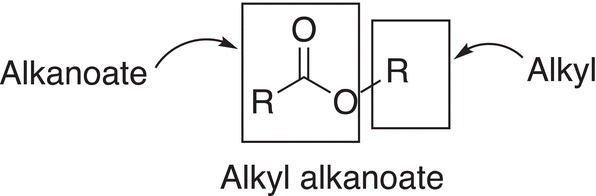
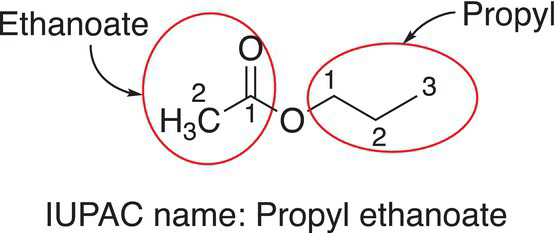
A specific example of the nomenclature of an ester is shown below.
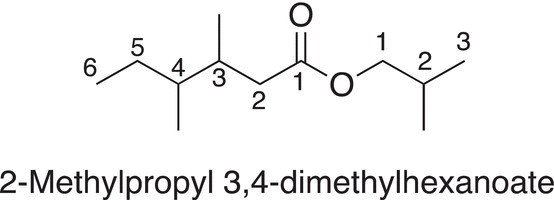
If there are substituents of either of both groups of an ester, the groups are assigned numbers based on the numbering starting from the groups bonded to the ester functionality, as illustrated in the example below.
Problem 3.16
i. Give line-angle structures for the following molecules.
1. Ethyl propanoate
2. Methyl butanoate
3. Butyl butanoate
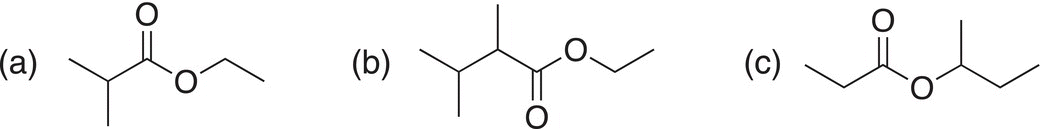
ii. Give IUPAC names for the molecules shown below.
3.9.2 Nomenclature of Cyclic Esters
Cyclic esters are also known as lactones. The IUPAC names of cyclic esters are based on the following general system: 2-oxacycloalkanone, as shown below. Note that this naming system is essentially based on an alkanone (which is assigned position #1, and the oxygen, which is named as an “oxa” gets position #2.
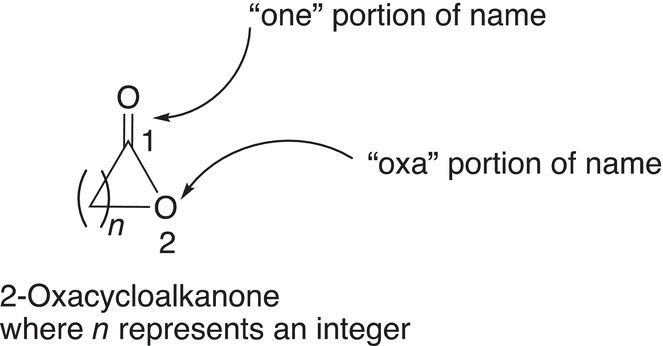
Thus, if n in the above example is 4, a six-membered ring is formed and named as 2-oxacyclohexanone. A more detailed illustration is shown below.
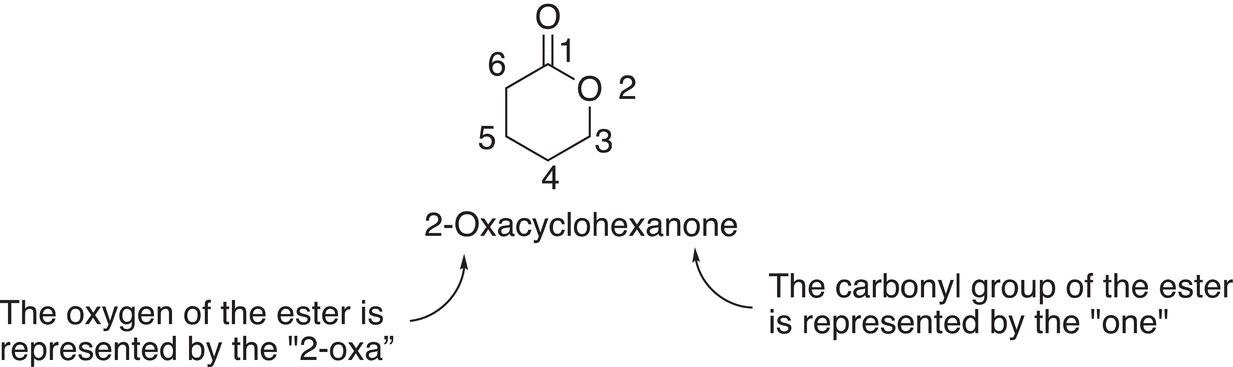
If there are substituents on the cyclic structure, it is identified based on the numbering system above; note that the numbering starts at the carbonyl carbon and proceeds in the direction of the oxygen of the ester. Examples giving the IUPAC names of different substituted lactones are shown below.
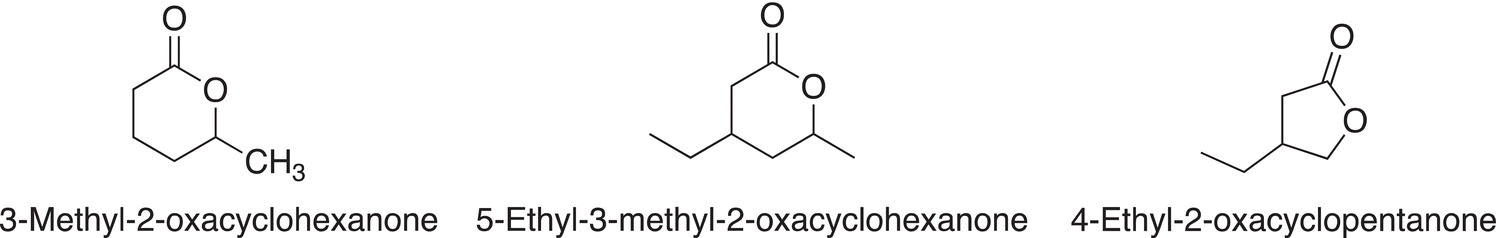
Common names are sometimes used for lactones. A very commonly used naming system for lactones is derived from the common names of the corresponding carboxylic acid in which Greek letters are assigned to the carbons adjacent to the carbonyl carbon of carboxylic acids. For the common names of lactones, the Greek letter is used to indicate the carbon that is bonded to the oxa oxygen in the cyclic structure, hence indicating the number of carbons that are in the lactone, examples are shown below.
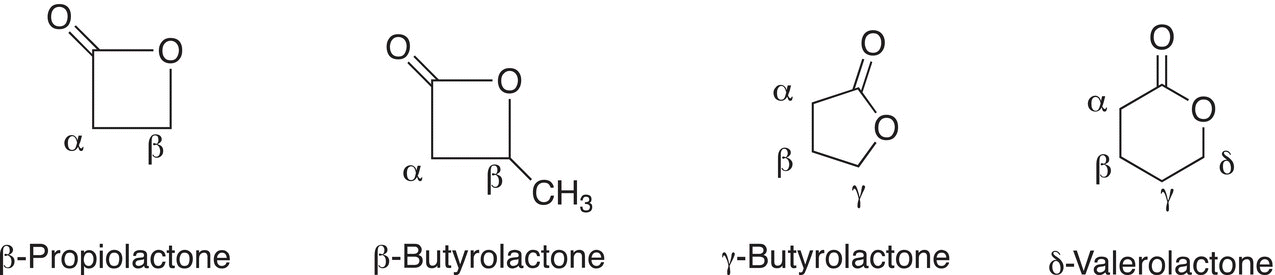
The first lactone has three carbons and is labeled as a β since the second carbon is bonded to the oxa oxygen. Likewise, the second is labeled β even though it has four carbons, but it is the second carbon that is bonded to the oxa oxygen.
Problem 3.17
i. Give the IUPAC names for the following molecules.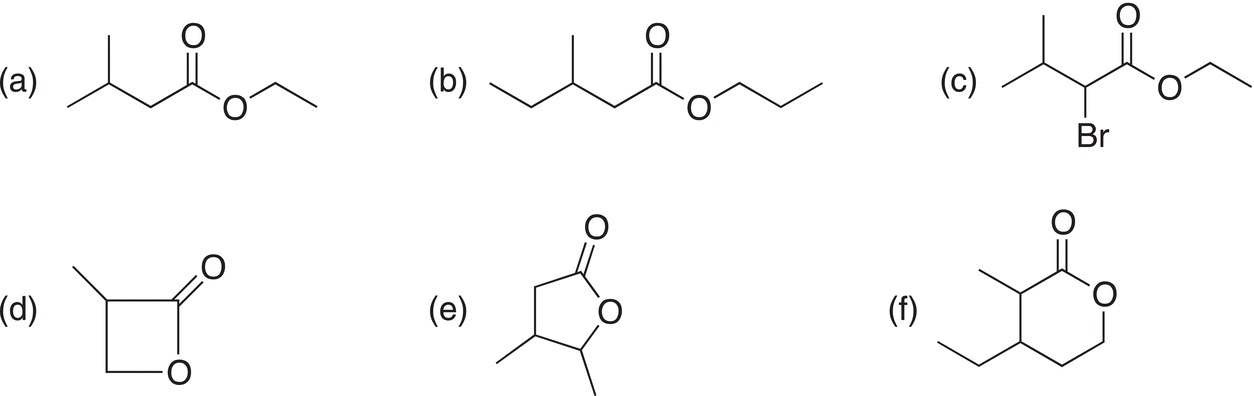
ii. Give structures for the following molecule.
a) Methyl butanoate |
b) Ethyl-2-methylpentanoate |
c) Methyl 2,3-dimethylhexenoate |
d) Ethyl 4-chlorobutanoate |
e) 2-Oxacyclopentanone |
f) 3-Methyl-2-oxacyclohexanone |
g) 4,4-Dimethyl-2-oxacyclopentanone |
h) 3-Ethyl-4-propyl-2-oxacyclohexanone |