Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
An Overview of the Reactions of Organic Chemistry
6.7 Substitution Reactions
A substitution reaction is simply a reaction in which one atom (or a group of atoms) in the reactant is substituted by another atom or group of atoms to form a product. We have seen this type of reaction in our general chemistry course, and an example is shown in Reaction (6-30).
(6-30)![]()
For this reaction, the NO3− anion (of silver nitrate) in the reactant is substituted for the chloride (the anion) from the sodium chloride in the reactant to form the product. Thus, for this substitution reaction, the nitrate anion replaces the chloride anion to form the product. Hence, this type of reaction is called a substitution reaction. Substitution reactions in organic chemistry are similar except the substitution takes place at a carbon atom. Since organic chemistry is the study of organic compounds that contain mostly carbon and hydrogen, most of the reactions that will be encountered throughout this course do not contain transition elements, but the atoms mentioned in Chapter 2 (functional groups). An example of a substitution reaction of an organic molecule is shown in Reaction (6-31). Note that the sodium is not involved in the substitution reaction, just the iodide and the bromine atom. The sodium ion is referred to as a spectator ion in this case.
(6-31)![]()
A similar analysis can be carried out for the reaction given in Reaction (6-31) as the reaction given in Reaction (6-30). For the substitution reaction involving 1-bromobutane and sodium iodide (Reaction 6-31), the iodide anion of sodium iodide replaces the bromine of 1-bromobutane to form the product 1-iodobutane. Since in organic chemistry, the emphasis is on the organic compounds in the reactant and the product, the other substitution product, sodium iodide, is considered to be inorganic and sometimes not included in the overall reaction. Other examples of substitution reactions are shown in Reactions (6-32) and (6-33).
(6-32)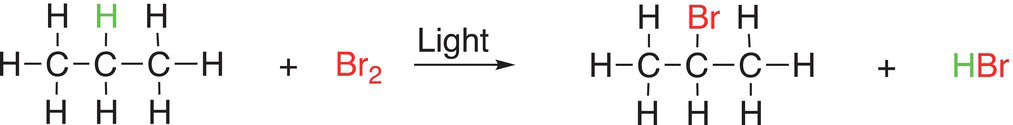
(6-33)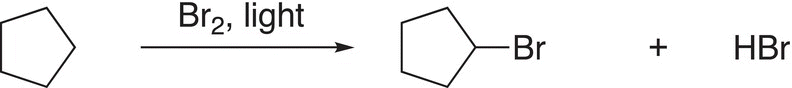
Note that for these reactions, a hydrogen atom in the reactant is substituted for a halogen (in this case, bromine) to form the organic product. This category of reactions (substitution reactions) can involve many different organic compounds, and as a result, substitution reactions form a major category of reactions in organic chemistry. At this point, students should be able to recognize a substitution reaction, compared to the other types of reactions covered so far and also be able to recognize the atom (or group of atoms) that is involved in the substitution and to predict the organic product. Problem 6.10 is designed to have students identify the atom (or group of atoms) involved in the substitution reactions.
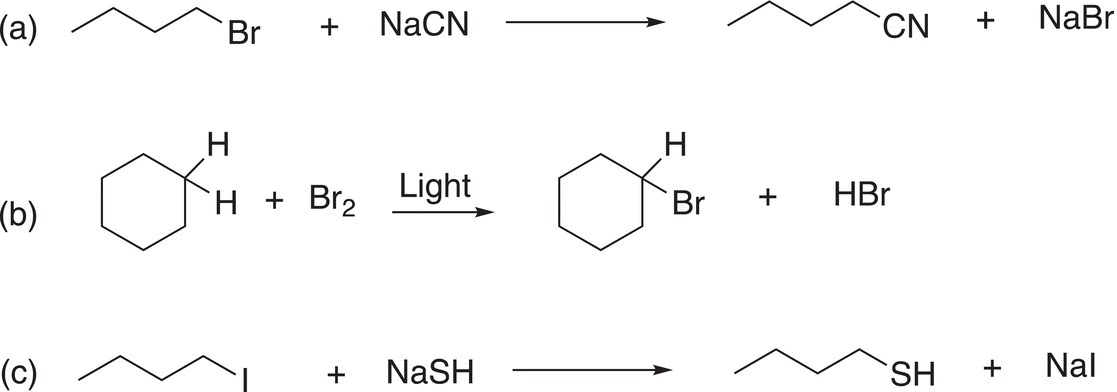
Problem 6.10
For the substitution reactions below, identify the atom (or group of atoms) in the reactants that are involved in the substitution reactions.
For the organic substitution reactions discussed thus far, substitution takes place at an sp3 carbon, but it is possible to have substitution reactions take place at an sp2 carbon, these types of reactions will be studied in detail in Chapters 16 and 17.