Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Acid—Base Reactions in Organic Chemistry
7.2 Lewis Acids and Bases
The Arrhenius, Brønsted-Lowry, and the Lewis definitions of acids and bases were covered in the previous chapter. In organic chemistry, the Lewis definition is more frequently used, compared to the other definitions. A Lewis acid is an electron pair acceptor. In order for a molecule to be an acid and be able to accept a pair of electrons, there must be a vacant orbital in which these electrons must be placed. Of course, the simplest Lewis acid is a proton (H+) since it has a vacant orbital. On the other hand, a Lewis base is an electron pair donor. Thus, Lewis bases have at least one unshared pair of electrons. A Lewis acid—base reaction involves an electron pair from the base being transferred to the vacant orbital of the acid, as illustrated in Reaction (7-1) for the reaction of methylamine (a Lewis base) with BF3, a Lewis acid.
(7-1)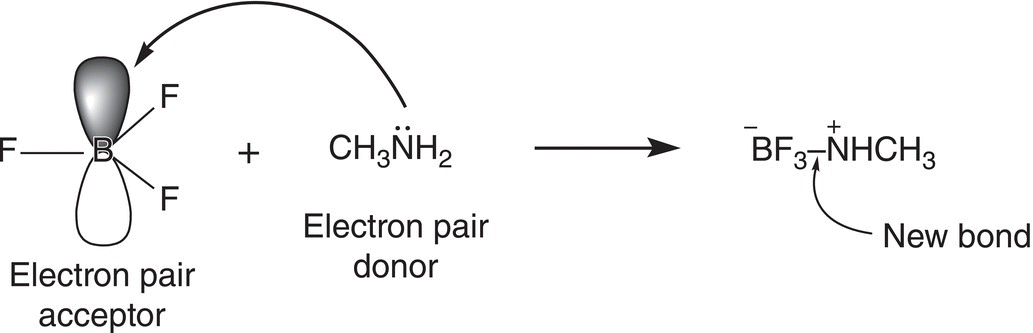
For the reaction given in Reaction (7-1), the lone pair of electrons on the methylamine forms a bond to BF3 using the vacant p orbital of BF3 to produce the product shown. Note, for the product for Reaction (7-1), the boron has acquired a formal negative charge due to the gain of a pair of electrons from the methylamine. On the other hand, the nitrogen has a positive charge since it has given up a pair of electrons to form a bond to boron.
A carbocation, which is sp2 hybridized and has a vacant p orbital, is also a Lewis acid and, as a result, can undergo a reaction with a Lewis base, such as the chloride anion since the chloride anion has four unshared pair of electrons. The reaction of a carbocation and the chloride anion is shown in Reaction (7-2). Acid—base reactions of this type will be encountered frequently throughout organic chemistry.
(7-2)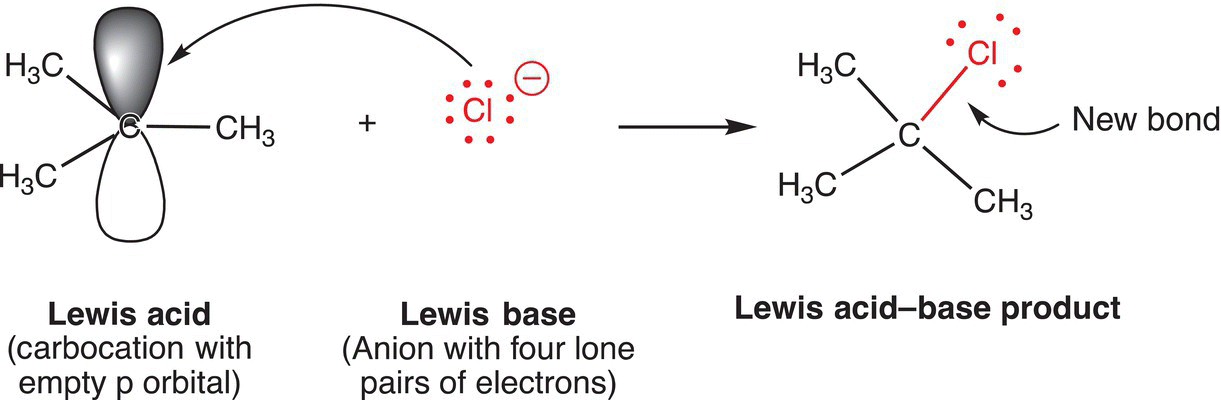
For the acid—base reaction given in Reaction (7-2), both reactants have formal charges of +1 and −1, respectively, and as a result, the product is neutral. The new bond is a result of using one pair of electrons from the chloride anion to form a bond using the vacant p orbital on the carbocation.
Problem 7.1
Which of the following are bases or acids? Explain your answer.
HI, H2O, NaCN, AlCl3, and KCl.
Ralph G. Pearson categorized acids and bases as hard and soft acids and bases (HSAB). Hard Lewis bases have small ionic radii and are typically highly solvated in a solvent; they are also relatively electronegative and nonpolarizable, NH2−, and OH− are two examples of hard Lewis bases. On the other hand, soft Lewis bases have large ionic radii, they are relatively large and polarizable, typically not highly solvated; the anions of P, S, and Cl are soft Lewis bases. Hard Lewis acids have small ionic radii and are highly solvated; the proton, H+ is considered to be the hardest acid and will be encountered frequently throughout organic chemistry. An example of a soft acid is Cu2+. Typically, hard acids bind strongly with hard bases and soft acids bind with soft bases.