Physical Chemistry Essentials - Hofmann A. 2018
Kinetics
6.1 Introduction
6.1.1 From Thermodynamics to Reaction Kinetics
The thermodynamic principles we have introduced in Sect. 2, and subsequently applied to a variety of systems, all had a common point of focus: they were targeting systems in equilibrium. A chemical reaction that has reached equilibrium still exhibits a forward and a reverse reaction, but the rates of both processes are equal, and since the reactions are of opposing direction, there is no net change.
In this part, we want to address the question of what happens to reactants in the course of time. This will also lead us to investigate what particular reaction pathways are engaged during a chemical reaction. The fundamental kinetics concepts will thus characterise a reaction with respect to
✵ time
✵ concentration
✵ temperature.
6.1.2 Spontaneous and Non-Spontaneous Reactions, Processes at Equilibrium
We have seen earlier (see Sect. 2.2.1) that the Gibbs free energy G provides a single criterion of spontaneity and equilibrium:
![]()
(2.44)
![]()
(2.42)
Importantly, the function G is based on state functions, and hence a state function itself. It was derived that:
✵ ΔG > 0 for a non-spontaneous process
✵ ΔG = 0 for a process at equilibrium
✵ ΔG < 0 for a spontaneous process.
With knowledge of the enthalpy of reaction and by calculating the entropy change ΔS sys (see Sect. 2.1.10), we can therefore predict whether or not a reaction will be spontaneous at a selected temperature.
For processes that are at equilibrium, the principle of Le Châtelier (Le Chatelier and Boudouard 1898) allows us to predict what happens to the position of the equilibrium when the conditions are changed:
A system at equilibrium, when subject to a disturbance, responds in a way that tends to minimise the effect of the disturbance.
Energy changes during a reaction
Consider the following reaction which describes the synthesis of ammonia from its elements:
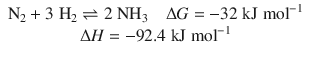
In this example, ΔG is the free energy change of the system, as 1 mol of nitrogen gas reacts with hydrogen to form ammonia. ΔG is a difference in free energy, i.e. the system has a lower free energy after the reaction proceeded to the right-hand side of the chemical equation. The fact that the free energy change is negative indicates that the reaction will proceed from the left to the right (albeit it requires pressures of 150—250 bar, temperatures of 300—550 °C and a catalyst—a technologically important process known as the Haber-Bosch process).
We see that the change of enthalpy during the reaction takes a much more negative value than the change of the free energy. This makes for two observations. First, the fact that the enthalpy change is negative indicates that the reaction is exothermic, i.e. 92.4 kJ mol−1 of heat are produced for every mol of nitrogen being transformed. Second, the question is what happens to the remaining energy of 60.4 kJ mol−1. This is energy “consumed” as the reaction proceeds from the left to right, because the disorder of the system is decreased. Whereas there are four molecules of starting products, there are only two molecules of end products; the system has taken a state with more order. Therefore, the entropy term T ⋅ ΔS accounts for 60.4 kJ for each mol of nitrogen transformed.
Note: Since the chemical reaction above describes the synthesis of ammonia from its elements, the change of enthalpy for this reaction is called the enthalpy of formation.
6.1.3 Stoichiometry and Molecularity
A stoichiometric equation is the simplest equation involving whole numbers of the molecules involved in the overall chemical reaction. For example:
![]()
![]()
![]()
Stoichiometric equations do not represent the kinetic equations! The molecularity of a reaction as calculated by adding the stoichiometric coefficients of the reactants does therefore in most cases not agree with the experimentally observed order of a reaction (see Sect. 6.2.2). However:
For an elementary reaction, the molecularity calculated as the number of reacting species in the stoichiometric equation, is the same as the order of the reaction.
An elementary reaction is defined as a single step reaction with a single transition state and no intermediates.
Therefore, more complex chemical reactions consist of a sequence of reactions each known as an elementary reaction. The concept of the transition state will be discussed in more detail in Sect. 6.5.2.
Reactions that involve
✵ One molecule are called unimolecular,
✵ Two molecules are called bimolecular, and
✵ Three molecules are called termolecular.