Physical Chemistry Essentials - Hofmann A. 2018
Kinetics
6.7 Polymerisation Kinetics
There are two main types of polymerisation processes that differ in their underlying reaction mechanism and the variation of the average molecular mass of the products over time (Table 6.2).
Table 6.2
Characteristics of the two main types of polymerisation reactions
|
Stepwise polymerisation |
Chain polymerisation |
✵ Any two monomers in the mixture can link together at any time. ✵ Growth of the polymer is not confined to chains already growing. ✵ Monomers are consumed early in the reaction. ✵ Average molecular mass of the product grows with time. |
✵ An activated monomer attacks another monomer and links to it. ✵ This unit then attacks another monomer and links to it. ✵ Long polymers are formed rapidly. ✵ The yield, but not the molecular mass of the product, increases with longer reaction times. |
6.7.1 Stepwise Polymerisation
For illustration of stepwise polymerisation processes, we will consider the polymerisation of polyamides (see Fig. 6.17). Examples of naturally occurring polyamides of technological importance are proteins which constitute wool and silk. Synthetic polyamides are typically made by stepwise polymerisation to produce materials such as Nylons and Aramids. Due to their high durability and strength, most polyamides are used in textiles, automotive applications, carpets and sportswear.
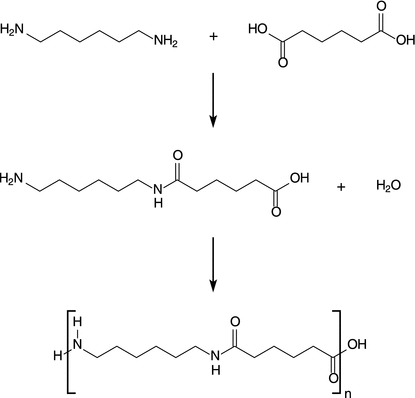
Fig. 6.17
Schematic illustration of the formation of Nylon 6-6 from hexane-1,6-diamine and hexanedioic acid (adipic acid). Instead of adipic acid, adipoyl chloride may be used
Here, two different monomers are joined together, and the resulting product is thus called a copolymer. In regular copolymers such as polyamides, polyesters and polyurethanes, the repeating unit consists of one of each monomer, so that they alternate in the chain. In the example shown in Fig. 6.17, each monomer has the same reactive group on both ends; the direction of the amide bond therefore alternates between each monomeric unit. This is different in natural polyamides (proteins), where the direction of the amide bond stays the same throughout.
The reaction rate in a stepwise polymerisation reaction depends on the concentration of ─NH2 (amine) and ─COOH (acid) groups:
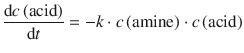
Because there is one amine group for each acid group (assuming that enough amine reactants are provided), it follows:
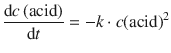
(6.53)
which constitutes a differential 2nd-order rate law.
If we assume that the rate constant for the reaction k remains constant with growing chain length, Eq. 6.53 can be integrated and yields (cf. Table 1.11):
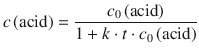
(6.54)
which is the integrated 2nd-order rate law. It provides a means to calculate the concentration of ’free’ (not yet condensed) acid groups at any time during the reaction.
The fraction of condensed acid groups, p, can then be calculated as the concentration of condensed acid groups, divided by the initial concentration of acid groups. By using the expression for the concentration of acid groups that are still available (Eq. 6.54), one obtains:
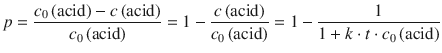
(6.55)
This equation can be arithmetically re-arranged to yield:
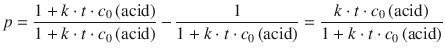
(6.56)
As a practical useful parameter, one defines the degree of polymerisation, <N>, as the average number of monomer residues per polymer molecule. <N> therefore indicates the average length of the polymer. It is calculated as the ratio between the initial concentration of acid, c 0(acid), and the concentration of acid end groups remaining non-condensed, c(acid). By using the relationship from Eq. 6.56, this yields Carothers’ equation (Carothers 1932) for stepwise polymerisation of linear polymers:
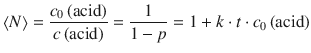
(6.57)
One can thus conclude that the average length <N> obtained from a stepwise polymerisation reaction grows linearly with time. The longer a stepwise polymerisation proceeds, the higher the average molecular mass of the product.
6.7.2 Chain Polymerisation
Many gas-phase reactions (for example the synthesis of hydrogen bromide from its elements, Sect. 6.6.1), but also liquid-phase polymerisations are chain reactions. In a chain reaction, an intermediate produced in one step generates another intermediate in the subsequent step. These intermediates are called chain carriers. In a radical chain reaction, the chain carriers are radicals, i.e. the intermediates possess unpaired electrons. Chain polymerisation occurs by addition of monomers to a rapidly growing polymer. The manufacture of frequently used plastics such as polyethylene (PE), polypropylene (PP), and polyvinyl chloride (PVC) is based on chain polymerisation (albeit these are mostly conducted catalytically, e.g. by using Ziegler—Natta catalysts). Mechanistically, the free radical mechanism consists of three stages: chain initiation, chain propagation, and chain termination (see Fig. 6.18).
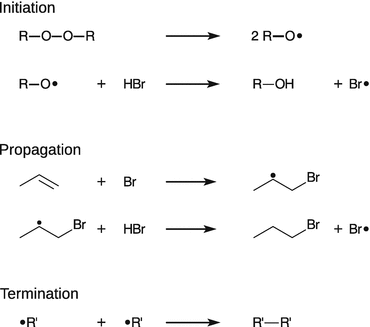
Fig. 6.18
Illustration of the three stages of a radical chain reaction that will result in polypropene. The radical starter in this example is a peroxide (R─O─O─R)
The central feature of the kinetic treatment of chain polymerisation reactions is that the rate is proportional to the square root of the initiator concentration:
![]()
(6.58)
In chain polymerisation processes, the kinetic chain length ν is defined as the number of monomer units consumed per number of activated centres produced in the initiation step. The kinetic chain length can be expressed in terms of the rate expressions: it is the rate of propagation of the chains (i.e. monomers are consumed at the rate with which chains propagate) divided by the rate of production of radicals. Without rigorously deriving this relationship, we appreciate that this yields the following expression for the kinetic chain length:
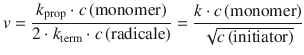
(6.59)
The final polymer produced by the chain mechanism may arise from mutual termination. In this case, the average number of monomers in the final polymer, <N>, is the sum of the numbers in the two combining chains. Since the average number of monomers in each chain is ν, we obtain:
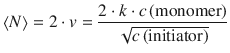
(6.60)
It follows that the slower the initiation of the chain (i.e. the lower the concentration of initiator and the slower the rate of initiation), the greater the kinetic chain length, and therefore the higher the average molecular mass of the polymer.