Physical Chemistry Essentials - Hofmann A. 2018
Kinetics
6.10 Diffusion Control
For diffusion-controlled reactions, we will need to consider the rate at which the reactants diffuse to each other. In Sect. 5.2.7, we learned that the diffusion coefficient D of a solute is given by the Stokes-Einstein equation:
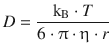
(5.48)
with η being the viscosity of the solution, and r the radius of the solute. Without rigorously deriving the following relationship, we appreciate that the rate constant of diffusion-controlled reactions can be calculated as follows:
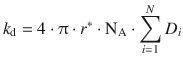
(6.74)
Here, the parameter r* describes the maximum distance the reactants may adopt such that a reaction can occur. The factor ![]() is the sum of the diffusion coefficients of all reactants.
is the sum of the diffusion coefficients of all reactants.
If we consider a bi-molecular reaction and substitute with the diffusion coefficients as calculated by the Stokes-Einstein relationship, we obtain:
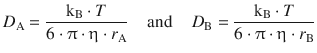
As an approximation, we assume that ![]() , so we can build the sum of the diffusion coefficients
, so we can build the sum of the diffusion coefficients
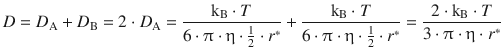
and then obtain for the rate constant of diffusion-controlled reactions (according to Eq. 6.74):
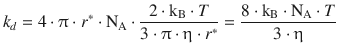
We remember that the product of Boltzmann’s and Avogadro’s constant equals the gas constant, kB·NA = R, and therefore arrive at the following expression for the rate constant of a bi-molecular diffusion-controlled reaction:
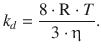
(6.75)
A notable observation from this approximation is the fact that the rate constant is independent of the identity of the reactants. Therefore, the rate constant for the diffusion-controlled reaction only depends on the temperature and the viscosity of the solvent!