Physical Chemistry Essentials - Hofmann A. 2018
The Chemical Bond
11.4 The Metallic Bond
The characteristic property of metals is their ability to conduct electric current. In contrast to the covalent bond where electrons are localised, the metallic bond is characterised by delocalised electrons. Notably, this delocalisation does not result in weaker bonding; the bond energies of metals are indeed of the same order as those of ionic crystals or covalent di-atomic molecules.
The ability to conduct electrons requires a reasonable spatial extension of the metal (as opposed to, for example, fairly isolated di-atomic gas molecules). In other words, in order for a metallic bond to exist, one requires a bulk assembly of atoms in a condensed phase. In order to understand the metallic bond, it will thus be necessary to consider the arrangement of large numbers of atoms in space. For illustration (Fig. 11.7), we start with a di-atomic molecule, say Li2 (which can be observed in the gas phase). Focussing on the outer shell only, the molecular orbital scheme for Li2 can be established by linear combination of the two 2s orbitals, leading to a σ and a σ* molecular orbital. Upon addition of a third and a fourth Li atom, the 2s orbitals of those atoms combine with the 2s orbitals of the initial Li atoms which leads to three and four molecular orbitals of different energies, respectively. For a large number of atoms (N), N molecular orbitals with slightly differing energies arise. If N = 6.022 · 1023, then the metal has 1 mol atoms and 6.022 · 1023 molecular orbitals. Whereas the energies of these individual orbitals are different, they are so densely situated that an energy band (here: s-band) is established in which the energy levels are pseudo-continuous.
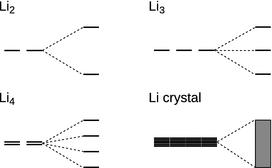
Fig. 11.7
The assembly of discrete atoms into bulk condensed matter in metals leads to generation of an electron band
In the case of a Li atom, there is one electron in the 2s orbital. This means that in metallic lithium, there are as many electrons as molecular orbitals arising from the 2s combination. These electrons populate the s-band; but because every orbital can harbour a maximum of two electrons, the band is only half-populated. Due to the energy levels in the band being pseudo-continuous, electrons can be take up indefinitely small amounts of energy and thus become highly mobile. The energy can be provided by an electric field, established by a potential difference, which leads to electrons travelling and thus conduction of an electric current.