Physical Chemistry Essentials - Hofmann A. 2018
Intermolecular Interactions
12.3 London Dispersion Force
Whereas the Keesom interactions and Debye forces require either a charged or polar species to be present, we now consider matter that entirely consists of neutral/non-polar atoms or molecules, such as e.g. helium or nitrogen gas. Since such gases can be condensed into liquids, there must be attractive interactions between those atoms/molecules in the absence of charged or polar species.
As illustrated in Fig. 12.2, an atom with spherical electron density is non-polar because it possesses no permanent dipole moment. However, this is only the view on average. At any individual moment in time, the electron density may not be spherically uniform but exhibit localised peaks. Such deformation of the spherical symmetry is mainly due to collisions between individual atoms. The non-uniform distribution of electrons constitutes a temporary dipole which can induce another temporary dipole in a neighbouring atom at appropriate distance and thus give rise to an attractive interaction, named the London dispersion force after the physicist Fritz London (1930).
For a pure substance, London showed that the interaction (potential) energy is given by
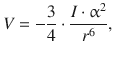
(12.6)
where α is the polarisability, and I the first ionisation potential. Similarly, for a mixture of substances A and B, the interaction is given by:
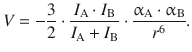
(12.7)
In contrast to the other dipole-based interaction types mentioned before (collectively termed van der Waals interactions), the London dispersion forces are always attractive. Independent of the relative orientation of two non-polar molecules, the induced dipoles will always possess compatible directions (since they are induced). The London dispersion forces are the weakest interactions among the van der Waals interactions. This is in agreement with macroscopic observations: the above-mentioned liquid helium and nitrogen boil at 4.2 and 77 K, respectively. These low temperatures suggest that only weak forces are holding the atoms/molecules together in the liquid state.