Physical Chemistry Essentials - Hofmann A. 2018
Intermolecular Interactions
12.4 van der Waals Interactions
Many molecules do not just engage one of the interactions discussed above, but their condensed states are held together by a combination of interactions involving dipoles. These combined interactions are termed van der Waals interactions, referring to all weaker forces between molecules and thus contrasting the stronger intermolecular interactions (Coulomb attraction, hydrogen bond). The attractive van der Waals interactions are often modelled by a potential that varies with sixth power of the distance—a relationship we have observed several times in above discussions (e.g. Debye force, London force).
Balanced by the universal repulsive force, which varies with the twelfth power of the distance, the van der Waals interactions result in the bonding interaction between two non-polar atoms/molecules. This interaction is often described by a Lennard—Jones potential and has been introduced in Sect. 11.5:
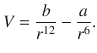
(11.22)
This superposition of the energies resulting from the attractive and repulsive forces results in a potential energy function that depends on the distance between the two molecules (or atoms) and that possesses a minimum at the equilibrium distance (see Fig. 11.8). This distance is the average distance the two particles maintain if there are no other forces acting on them.
By way of example, the potential energy of two argon atoms approaching other decreases as they are brought closer together. However, the minimum energy attained at the equilibrium distance is approx. −1.3 kJ mol−1 the value of which is less than the thermal energy at ambient temperature (E therm = R · T ≈ 2.5 kJ mol−1), and thus not enough to hold the two atoms together. These non-bonding attractions enable argon to exist as a liquid and solid at low temperatures (when the potential energy is larger than the thermal energy). However, at ambient temperature, the potential energy due to van der Waals interactions is not enough to withstand disruptions caused by thermal energy, so argon exists as a gas under ambient conditions.
Table 12.2 shows estimates of the contributions of the various types of van der Waals forces that act between different types of molecules. This comparison highlights the importance of the ubiquitous dispersion forces on the one hand, even in cases of polar molecules (high dipole moment).
Table 12.2
Contributions of the various types of van der Waals forces in select molecules
|
Boiling point (°C) |
Dipole moment (D) |
Polarisability (10−40 C m2 V−1) |
Interaction |
|||
Dipole—induced dipole (%) |
Dipole—dipole (%) |
Dispersion (%) |
||||
Ar |
−186 |
0 |
1.85 |
0 |
0 |
100 |
CO |
−190 |
0.117 |
2.20 |
0 |
0 |
100 |
HCl |
−84 |
1.08 |
2.93 |
4.2 |
14.4 |
81.4 |
NH3 |
−33 |
1.47 |
2.47 |
5.4 |
44.6 |
50.0 |
H2O |
100 |
1.85 |
1.65 |
4.0 |
77.0 |
19.0 |