Physical Chemistry Essentials - Hofmann A. 2018
Intermolecular Interactions
12.5 The Hydrogen Bond
With hydrogen only possessing one electron, the nucleus becomes partially unshielded when the atom engages in a covalent bond. The ’partial proton’ can then interact directly with a nearby atom that possesses a lone pair of electrons, such as oxygen, nitrogen, fluorine or chlorine. The existence of hydrogen bonds has drastic effects on properties of substances and is of tremendous importance for the folding and properties of biological macromolecules. Depending on geometry and environmental conditions, the potential energy of a hydrogen bond is between 5 and 30 kJ mol−1, which makes it much stronger than van der Waals interactions, but weaker than covalent or ionic bonds. The hydrogen bond extends from the hydrogen bond acceptor (the atom that has a lone pair of electrons) to the hydrogen bond donor (the electronegative atom to which the hydrogen atom is covalently bound).
For example, the boiling points of liquids in a series of homologues increases with polarisability (which results in stronger London dispersion forces; see Sect. 12.3). However, in the series shown in Table 12.3, H2O possesses a substantially higher boiling point than the homologous compounds, while indeed it would be expected to have the lowest boiling point based on the London dispersion forces. Despite the low polarisability of H2O, the existence of intermolecular hydrogen bonds leads to much stronger interactions between water molecules and thus results in the much higher boiling point.
Table 12.3
Comparison of boiling points of a homologue series. The polarisability increases with the heavier group VI atoms. This leads to stronger dispersion forces and thus higher boiling points. However, the strong hydrogen bonds in water give rise to a substantial increase in interaction energy between H2O molecules, leading to an unusually high boiling point
|
Liquid |
Boiling point (K) |
Polarisability α |
Dipole moment μ (D) |
H-bonds possible |
H2O |
373 |
|
1.8 |
+ |
H2S |
213 |
1.1 |
— |
|
H2Se |
231 |
0.4 |
— |
|
H2Te |
271 |
0.2 |
— |
Each H2O molecule contains two hydrogen atoms and two lone pairs. In water, the number of hydrogen bonds is therefore maximised by a tetrahedral arrangement of hydrogen atoms around the oxygen atoms. In the hexagonal structure of ice, each oxygen atom is surrounded by a distorted tetrahedron of hydrogen atoms that form bridges to the oxygen atoms of adjacent water molecules. Importantly, the hydrogen atoms are not located at the same distance from the two oxygen atoms they connect (see Fig. 12.3); the shorter distance is indicative of a covalent O—H bond, and the longer distance constitutes the hydrogen bond. The hexagonal structure of ice is the form of all natural snow and ice on Earth (note the sixfold symmetry in ice crystals grown from water vapour). The packing of H2O molecules in this cage-like structure is expanded as compared to the packing of molecules in liquid water. Therefore, ice is less dense than liquid water, which explains why it floats on the liquid.
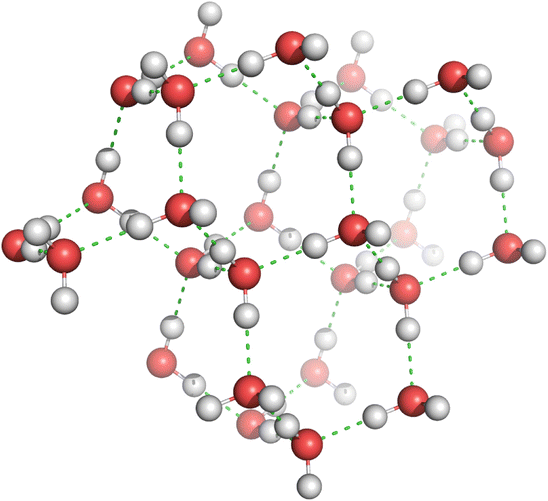
Fig. 12.3
The hexagonal structure of ice. Oxygen atoms are shown in red and hydrogen atoms in grey. Hydrogen bonds are indicated by green dashed lines. The distance of the covalent O—H bond measures 1.01 Å and that of the hydrogen bond 1.74 Å, respectively
