Chemistry: A Self-Teaching Guide - Post R., Snyder C., Houk C.C. 2020
Solids
Look around you. What do you see? Lots of visible things, such as buildings, tables, chairs, wires, windows, and trees. This chapter is an introduction to the structure and properties of the solid state.
The chapter will let you compare solids and gases, and will explore how bonding between particles affects the properties of solids.
We will study the shapes of solids, that is, the different ways atoms, ions, and molecules arrange themselves in three dimensions. Apart from shape, four types of solids have distinctive properties dependent upon the type of bonding between particles. You already know that different substances have different thermal properties. We will show how a specific thermal property may be used to calculate approximate atomic weights of pure metals. Finally, we will discuss the relationship between the size of an ion and its parent atom, and the sizes of atoms and ions that contain the same number of electrons.
OBJECTIVES
After completing this chapter, you will be able to
· recognize and apply or illustrate: specific heat, crystal lattice, allotrope, polymorphic, heat of fusion, melting point, sublimation, diffraction, isoelectronic, amorphous;
· explain the differences between “glasses” and crystals;
· explain the differences between gases and solids;
· explain why an ion has a different radius than its parent atom;
· determine if a group of ions and atoms is isoelectronic;
· calculate the approximate atomic weight of a pure metal when given the specific heat of the metal;
· calculate the amount of heat required to melt a solid knowing the heat of fusion of the solid;
· locate the melting point and other properties of a solid on a graph that shows what happens when a solid is heated;
· describe the differences in properties, bonding, and structure among ionic, covalent, molecular, and metallic solids.
![]() In Chapter 8, the kinetic molecular theory of gases described gases as molecules that move rapidly and readily to conform to the shape of the container. A solid, on the other hand, will not conform readily to the shape of its container unless it is forced to do so, for example if it is crushed to a powder. The atoms or molecules of a solid are fixed in place and do not move; they only vibrate within the confines of their positions in well-defined structures.
In Chapter 8, the kinetic molecular theory of gases described gases as molecules that move rapidly and readily to conform to the shape of the container. A solid, on the other hand, will not conform readily to the shape of its container unless it is forced to do so, for example if it is crushed to a powder. The atoms or molecules of a solid are fixed in place and do not move; they only vibrate within the confines of their positions in well-defined structures.
Ice cubes and coal lumps need to be crushed to conform to the walls of a container. Are ice cubes and coal lumps gases or solids? __________
Answer: solids
![]() The forces attracting one gas molecule to another are negligible (except at low temperatures and high pressures). These are the van der Waals forces discussed in Chapter 8. In solids, the forces of attraction between atoms and molecules are relatively strong, and a great deal of energy is required to overcome these forces. In gases, most of the volume (theoretically all of the volume) is the space between the molecules. Gases are easily compressed. Changes in temperature and pressure have comparatively little effect on the volume of a solid. Even extreme pressure causes only a slight reduction in the volume of solids.
The forces attracting one gas molecule to another are negligible (except at low temperatures and high pressures). These are the van der Waals forces discussed in Chapter 8. In solids, the forces of attraction between atoms and molecules are relatively strong, and a great deal of energy is required to overcome these forces. In gases, most of the volume (theoretically all of the volume) is the space between the molecules. Gases are easily compressed. Changes in temperature and pressure have comparatively little effect on the volume of a solid. Even extreme pressure causes only a slight reduction in the volume of solids.
We know that there is a lot of space between molecules or atoms in a gas. Based on the above information, would you expect a lot or a little space between the molecules or atoms of a solid? ____________________
Answer: little
![]() Melting is defined as changing from the solid state to the liquid state. The temperature at which both the liquid and solid state are present at the same time is known as the melting point of the solid. If a liquid is changing to a solid, this same melting point temperature is also called the freezing point. Each solid has a specific, unique temperature as its melting point.
Melting is defined as changing from the solid state to the liquid state. The temperature at which both the liquid and solid state are present at the same time is known as the melting point of the solid. If a liquid is changing to a solid, this same melting point temperature is also called the freezing point. Each solid has a specific, unique temperature as its melting point.
1. Melting is the change of state from which to which: solid to liquid or liquid to solid? _______________
2. Is the temperature at which a liquid changes to a solid its melting point or its freezing point? ________________
Answer: (a) solid to liquid; (b) freezing point
![]() Molecules on the surface of some solids escape from the solid and enter a gaseous state directly without entering the liquid state first. These solids exhibit a measurable vapor pressure. One of the best known examples of this is dry ice, which is solid carbon dioxide. The phenomenon of a solid changing directly to the gaseous state is known as sublimation. The liquid state is bypassed.
Molecules on the surface of some solids escape from the solid and enter a gaseous state directly without entering the liquid state first. These solids exhibit a measurable vapor pressure. One of the best known examples of this is dry ice, which is solid carbon dioxide. The phenomenon of a solid changing directly to the gaseous state is known as sublimation. The liquid state is bypassed.
Solid mothballs become smaller over a period of time without the appearance of any liquid. The distinctive odor of mothballs in the air indicates that some of the solid has vaporized. Wet clothes hung out on a clothesline freeze if the outside temperature remains below the freezing point! Although the temperature remains below the freezing point, the clothes do dry. The ice crystals go directly to the gaseous state over a period of time. You may also have noticed that ice cubes shrink when left in the freezer for a long time. Have the mothballs, ice cubes, and ice crystals in the frozen clothes melted or sublimed? __________
Answer: sublimed
CRYSTALS AND THEIR SHAPES
![]() Many naturally occurring solids have very distinct geometrical shapes. Table salt, NaCl, looks like cubes. Calcite, CaCO3, looks like a rectangular solid. Alum, KAl(SO4)2 • 12H2O, looks like two pyramids base to base. A solid that exists in a definite three-dimensional geometrical shape is known as a crystalline solid or crystal. Crystalline solids have a characteristic shape regardless of the size of the piece. There are seven basic crystal shapes that have been identified in naturally occurring solids. They are shown on page 206, along with some variations of the basic shapes, giving a total of 14 arrangements, or crystal lattices, in which crystalline solids occur.
Many naturally occurring solids have very distinct geometrical shapes. Table salt, NaCl, looks like cubes. Calcite, CaCO3, looks like a rectangular solid. Alum, KAl(SO4)2 • 12H2O, looks like two pyramids base to base. A solid that exists in a definite three-dimensional geometrical shape is known as a crystalline solid or crystal. Crystalline solids have a characteristic shape regardless of the size of the piece. There are seven basic crystal shapes that have been identified in naturally occurring solids. They are shown on page 206, along with some variations of the basic shapes, giving a total of 14 arrangements, or crystal lattices, in which crystalline solids occur.
If a large crystal is cleaved (split), would the smaller pieces still be crystals? _______________________
Answer: yes (Crystalline solids have a characteristic shape regardless of the size of the pieces. This is how a jeweler cuts a large diamond into several smaller ones.)
The seven crystal systems and their variations are determined by the arrangement of atoms, ions, and molecules within the crystals. The arrangement of atoms within the crystal is known as the crystal lattice. We can determine this arrangement by a process called X-ray diffraction. Planes of atoms and ions that make up the crystal diffract X-rays similar to the way a mirror reflects light. X-ray diffraction analysis of crystals is possible because the wavelengths of X-rays are very close in size to the diameters of the atoms. Visible light has much longer wavelengths than X-rays; therefore, the individual atoms within the crystal lattice will not diffract normal light. When X-rays are used, the resulting diffraction may be photographed or sent directly to a computer for analysis and mathematical determination of the structure.
We now discuss some more properties of crystalline and noncrystalline solids.
![]() Some substances can assume more than one crystalline shape (as shown in the figures below). Sulfur, for example, can be found as rhombic crystals or monoclinic crystals. A substance is polymorphic if it exists in more than one crystalline form. Calcium carbonate, CaCO3, occurs in nature with either the hexagonal structure or the rhombic structure. Is CaCO3 an example of a polymorphic solid? _______ Explain why or why not. _________________
Some substances can assume more than one crystalline shape (as shown in the figures below). Sulfur, for example, can be found as rhombic crystals or monoclinic crystals. A substance is polymorphic if it exists in more than one crystalline form. Calcium carbonate, CaCO3, occurs in nature with either the hexagonal structure or the rhombic structure. Is CaCO3 an example of a polymorphic solid? _______ Explain why or why not. _________________
Answer: yes; it exists in more than one crystalline form.
![]() When a chemical element is found to be polymorphic, the different crystalline forms of the element are known as allotropes (or allotropic forms) of the element. Carbon is a polymorphic element. Graphite and diamond are both allotropic forms of carbon.
When a chemical element is found to be polymorphic, the different crystalline forms of the element are known as allotropes (or allotropic forms) of the element. Carbon is a polymorphic element. Graphite and diamond are both allotropic forms of carbon.
1. Sulfur is an element that occurs in two forms. Thus, rhombic sulfur and monoclinic sulfur are ________________ of sulfur.
2. White phosphorus, red phosphorus, and yellow phosphorus are crystalline forms of phosphorus; therefore, phosphorus is called a ______________________ substance.
Answer: (a) allotropes; (b) polymorphic
![]() Not all solids are crystalline. A solid that does not have a definite crystalline shape is said to be amorphous. Glass is a common example of an amorphous solid. It is a solid, but it is not crystalline.
Not all solids are crystalline. A solid that does not have a definite crystalline shape is said to be amorphous. Glass is a common example of an amorphous solid. It is a solid, but it is not crystalline.
If a piece of glass were broken into smaller pieces, could you predict the shape of those pieces? Why or why not? ___________________________
Answer: No, because glass is not crystalline, it has no definite geometrical form, such as one of the basic crystal shapes.
![]() The entire category of solid substances that appear to be crystalline but are not is called glasses. Glasses have several characteristics that differ from crystals. Crystals have sharp melting points. Melting destroys the attractions that give solids their rigid structure. Below the sharp melting point, crystals are solids. Above the sharp melting point, crystals become liquids. Glasses have relatively large melting ranges instead of a sharp melting point. Glasses generally soften over several degrees of increasing temperature before finally becoming liquid.
The entire category of solid substances that appear to be crystalline but are not is called glasses. Glasses have several characteristics that differ from crystals. Crystals have sharp melting points. Melting destroys the attractions that give solids their rigid structure. Below the sharp melting point, crystals are solids. Above the sharp melting point, crystals become liquids. Glasses have relatively large melting ranges instead of a sharp melting point. Glasses generally soften over several degrees of increasing temperature before finally becoming liquid.
Ice melts at 0°C. It does not become soft before melting. Is ice more likely to be a glass or a crystalline solid? __________
Answer: crystalline solid
![]() Crystals cleave (split) along planar (flat) surfaces when struck. Even when broken, crystals retain their basic structural shape. Glasses, on the other hand, fracture to form uneven surfaces when struck. Breakage is not along any definite pattern.
Crystals cleave (split) along planar (flat) surfaces when struck. Even when broken, crystals retain their basic structural shape. Glasses, on the other hand, fracture to form uneven surfaces when struck. Breakage is not along any definite pattern.
A jeweler can split a gemstone with a jeweler's chisel. The surfaces along the split are flat and the cut is clean. Is the gemstone a crystal or a glass? __________
Answer: crystal
![]() When heated, a solid substance becomes soft and pliable several degrees before actually melting. Is the substance likely to be crystalline? __________
When heated, a solid substance becomes soft and pliable several degrees before actually melting. Is the substance likely to be crystalline? __________
Answer: no (Crystals have sharp melting points and do not soften before melting.)
![]() In crystals, the strengths of the chemical bonds between the atoms, ions, or molecules are all the same. This accounts for their very sharp melting point. In glasses, the strengths of the bonds vary throughout the solid. This accounts for the wide melting point range of glasses. In which class of solids (glasses or crystals) would it be possible for some bonds to be broken at one temperature while other bonds would not be broken until a slightly higher temperature was reached?_______________
In crystals, the strengths of the chemical bonds between the atoms, ions, or molecules are all the same. This accounts for their very sharp melting point. In glasses, the strengths of the bonds vary throughout the solid. This accounts for the wide melting point range of glasses. In which class of solids (glasses or crystals) would it be possible for some bonds to be broken at one temperature while other bonds would not be broken until a slightly higher temperature was reached?_______________
Answer: glasses (With bonds of varying energies, some bonds would be broken at lower temperatures than other bonds.)
HEAT OF FUSION
![]() When crystalline solids melt, the crystal lattice is broken down. The solids lose their shape and become fluid. The energy required to change a solid to a liquid while remaining at the melting point temperature is called the heat of fusion. The energy required to change a block of ice at 0°C to water at 0°C is known as the _____________ for H2O.
When crystalline solids melt, the crystal lattice is broken down. The solids lose their shape and become fluid. The energy required to change a solid to a liquid while remaining at the melting point temperature is called the heat of fusion. The energy required to change a block of ice at 0°C to water at 0°C is known as the _____________ for H2O.
Answer: heat of fusion
![]() Heat of fusion can be expressed as either calories per gram or calories per mole. The calorie (cal) is a unit of energy in the form of heat. The heat of fusion of H2O is 80 calories per gram. The energy required to melt a 1 gram ice cube at 0°C to water at 0°C is __________________
Heat of fusion can be expressed as either calories per gram or calories per mole. The calorie (cal) is a unit of energy in the form of heat. The heat of fusion of H2O is 80 calories per gram. The energy required to melt a 1 gram ice cube at 0°C to water at 0°C is __________________
Answer: 80 calories (Since the heat of fusion for H2O is 80 calories per gram, it takes 80 calories to melt 1 gram of ice.)
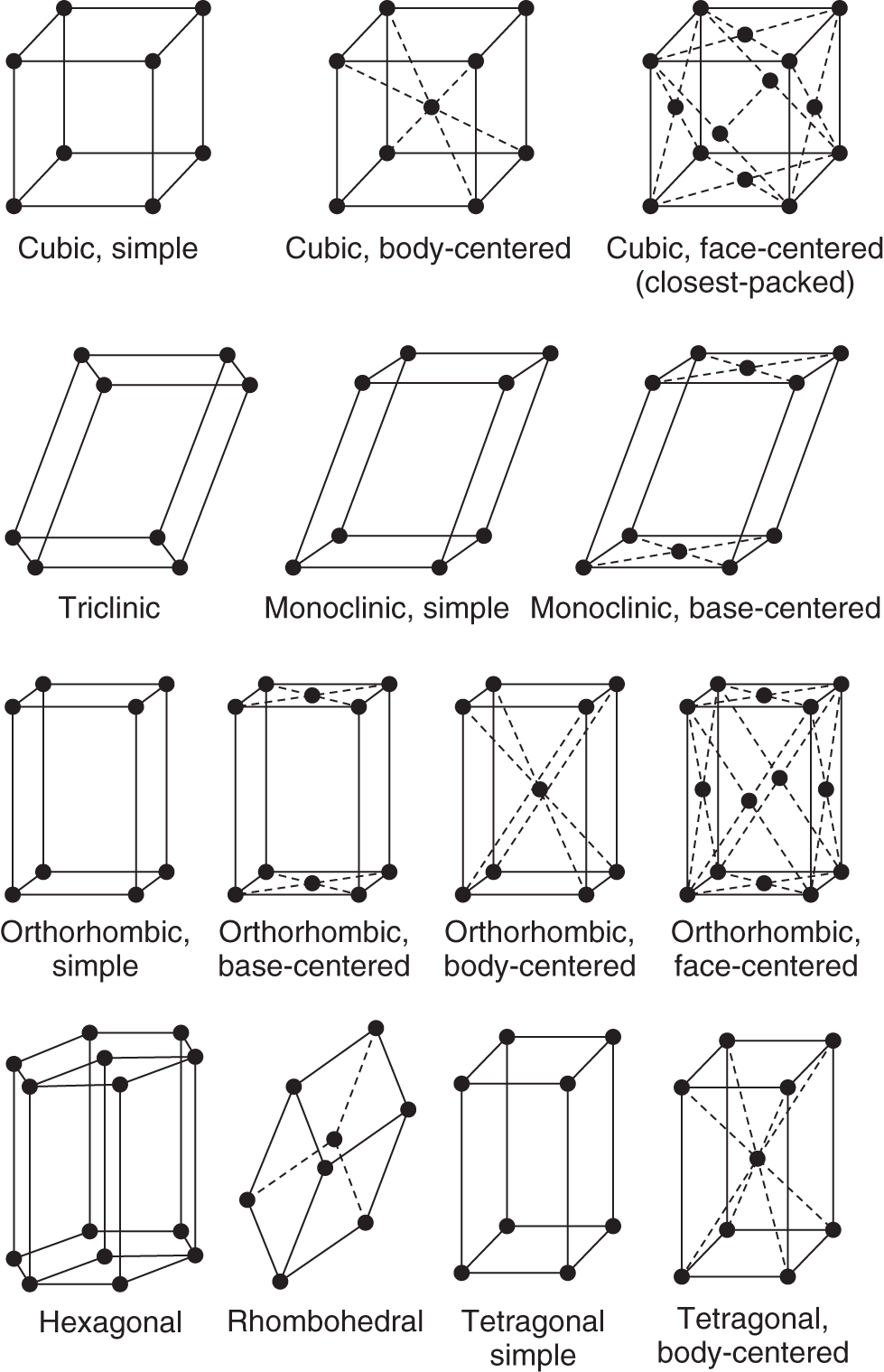
![]() The heat of fusion can be graphically shown by plotting temperature against the energy requirement.
The heat of fusion can be graphically shown by plotting temperature against the energy requirement.
Remember that the heat of fusion is the energy required to liquefy a solid at the melting point. When a solid is heated, its temperature rises until the melting point. At the melting point the temperature remains constant until all the solid has melted. After melting is completed, the temperature of the substance (now totally liquid) rises as more heat is applied. Which part of the graph shows the heat of fusion? (A, B, C) ______________
Answer: B (because the temperature remains constant at the heat of fusion)
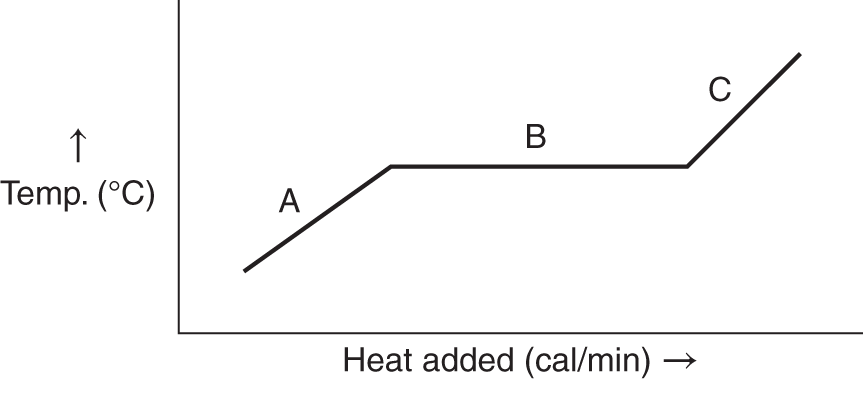
![]() If the graph below represents H2O, at what point on the temperature scale should the melting point (0°C) be placed? (A, B, C, D, E) __________
If the graph below represents H2O, at what point on the temperature scale should the melting point (0°C) be placed? (A, B, C, D, E) __________
Answer: C (the temperature at which the heat of fusion is observed)
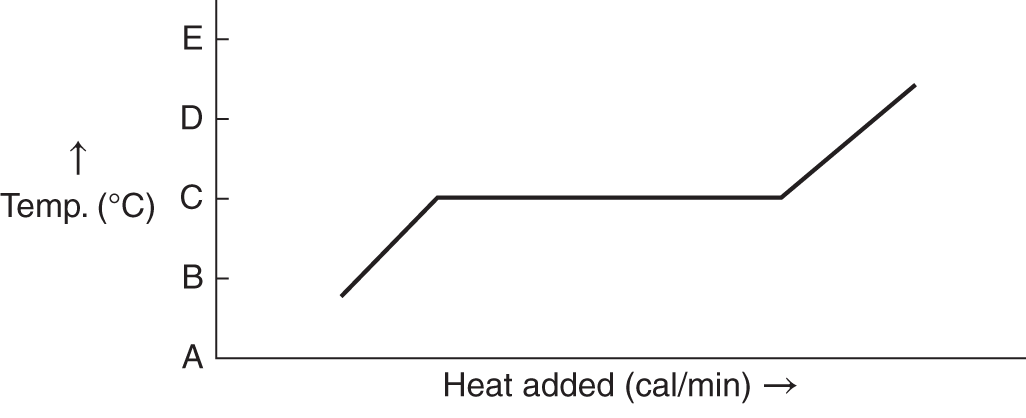
![]() The graph below represents heat applied to a substance such as H2O or some other crystalline substance.
The graph below represents heat applied to a substance such as H2O or some other crystalline substance.
For each of the indicated points, say whether the substance is solid, liquid, or both solid and liquid.
1. at point A ____________
2. at point B ______________
3. at point C _________________
Answer: (a) solid; (b) both solid and liquid (energy as the heat of fusion is being used to melt the solid); (c) liquid
![]() The next graph represents the reverse process. Heat is removed from a system and a liquid freezes into a solid. In this case, the heat of fusion energy must be removed for the liquid at the freezing point to become solid at the freezing point.
The next graph represents the reverse process. Heat is removed from a system and a liquid freezes into a solid. In this case, the heat of fusion energy must be removed for the liquid at the freezing point to become solid at the freezing point.
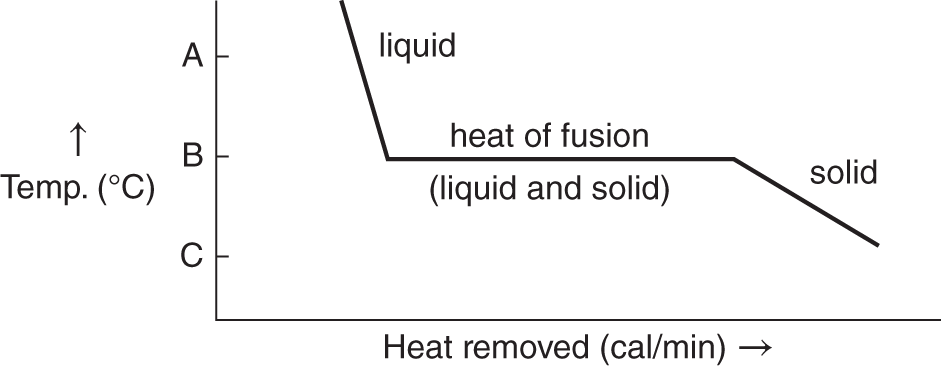
The freezing point (same temperature as the melting point) is indicated by which letter on the temperature scale? (A, B, C) ________
Answer: B
![]() Remember that the heat of fusion for H2O is 80 calories per gram, with the temperature remaining at the melting point. Based on this information, how many calories of heat do you think must be removed to freeze 1 gram of water if the temperature remains at the freezing point? __________
Remember that the heat of fusion for H2O is 80 calories per gram, with the temperature remaining at the melting point. Based on this information, how many calories of heat do you think must be removed to freeze 1 gram of water if the temperature remains at the freezing point? __________
Answer: 80 calories (The heat of fusion applies to both melting and freezing.)
![]() For the moment, ignore the heat of fusion in the graph below. Before the heat of fusion, what happens to the temperature of the solid when heat is added: is it increased, decreased, or unchanged? __________
For the moment, ignore the heat of fusion in the graph below. Before the heat of fusion, what happens to the temperature of the solid when heat is added: is it increased, decreased, or unchanged? __________
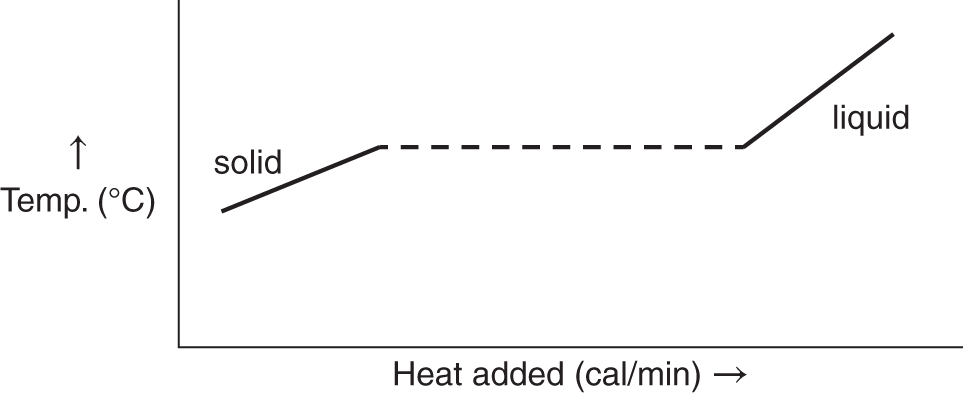
Answer: increased
SPECIFIC HEAT
![]() Each solid substance as well as each liquid requires a different quantity of heat added to increase the temperature by a given amount. To raise the temperature of 1 gram of solid iron 1° on the Celsius scale requires 0.108 calories. To raise the temperature of 1 gram of solid lead 1° on the Celsius scale requires 0.0306 calories.
Each solid substance as well as each liquid requires a different quantity of heat added to increase the temperature by a given amount. To raise the temperature of 1 gram of solid iron 1° on the Celsius scale requires 0.108 calories. To raise the temperature of 1 gram of solid lead 1° on the Celsius scale requires 0.0306 calories.
Which substance requires more heat, measured in calories, to raise its temperature 1°: solid lead or solid iron? __________
Answer: solid iron (Iron requires more than three times as much heat as lead to raise the temperature 1°C for each gram.)
![]() The calories required to raise the temperature of 1 gram of a substance by 1°C is called the specific heat of the substance. The specific heat of copper is 0.093 calories per gram. The specific heat of aluminum is 0.219 calories per gram. Which has the lower specific heat, aluminum or copper? __________
The calories required to raise the temperature of 1 gram of a substance by 1°C is called the specific heat of the substance. The specific heat of copper is 0.093 calories per gram. The specific heat of aluminum is 0.219 calories per gram. Which has the lower specific heat, aluminum or copper? __________
Answer: copper
![]() Specific heat is useful because it is related to atomic weight.
Specific heat is useful because it is related to atomic weight.
![]()
The atomic weight found by this formula is a reasonable approximation of the actual atomic weight of solid elements. The formula shows a relationship between __________ and __________.
Answer: atomic weight; specific heat
![]() The formula is useful for determining approximate atomic weights and identifying unknown substances.
The formula is useful for determining approximate atomic weights and identifying unknown substances.
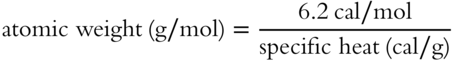
For example, the specific heat for iron is 0.108 cal/g.
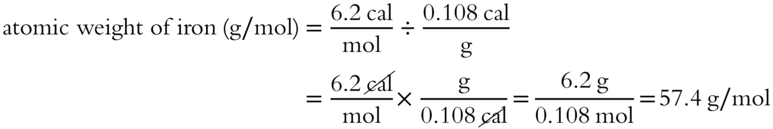
Since the actual atomic weight of iron is 55.8 grams/mol, the result is an approximate atomic weight.
What is the approximate atomic weight (to the nearest tenth) of nickel if it has a specific heat of 0.105 cal/g? __________
Answer: 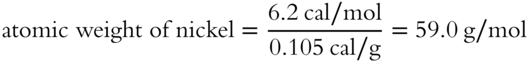
(The actual atomic weight of nickel is 58.71 g/mol.)
![]() A solid element is found to have a specific heat of 0.0565 cal/g. Determine the approximate atomic weight of the element (to the nearest tenth). ____________
A solid element is found to have a specific heat of 0.0565 cal/g. Determine the approximate atomic weight of the element (to the nearest tenth). ____________
Answer: 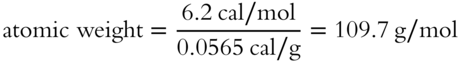
atomic weight = 109.7 g/mol
![]() Look at the periodic table and determine which solid element is closest in atomic weight to 109.7 g/mol. ________________
Look at the periodic table and determine which solid element is closest in atomic weight to 109.7 g/mol. ________________
Answer: silver (The closest element to an atomic weight of 109.7 is silver, Ag, with an actual atomic weight of 107.87 g/mol. Another possibility is cadmium, Cd, with an atomic weight of 112.41 g/mol. Further chemical analysis is necessary to positively identify the element. This is a good approximation, however, and limits the choices.)
Now that you have learned some of the properties of solid substances, we will examine the properties of four general types of solids and cite commonly occurring examples of each.
TYPES OF SOLIDS
![]() The melting points of different solids vary with the differences in interparticle attraction found among the various solids. Crystalline solids are classified in four categories based upon differences in interparticle attraction and in the nature of the particles (atoms, ions, or molecules) that make up the crystals. The four categories are ionic solids, covalent solids, molecular solids, and metallic solids. (You should remember the terms ionic and covalent as two types of chemical bonds discussed in Chapter 3.)
The melting points of different solids vary with the differences in interparticle attraction found among the various solids. Crystalline solids are classified in four categories based upon differences in interparticle attraction and in the nature of the particles (atoms, ions, or molecules) that make up the crystals. The four categories are ionic solids, covalent solids, molecular solids, and metallic solids. (You should remember the terms ionic and covalent as two types of chemical bonds discussed in Chapter 3.)
Ionic solids are made up of ions held together by ionic bonds. Negative and positive ions are bonded together in a regular arrangement of negative to positive to negative to positive and so on, throughout the neatly stacked arrangement within the crystal lattice. The strong electrostatic attraction between the ions is responsible for the hardness and generally high melting points of ionic solids. Examples of ionic solids are NaCl and KCl.
1. In an ionic solid is the attraction between ions weak or strong? __________
2. Would you expect an ionic solid to have a low or high melting point? _____________________
3. Would you expect an ionic solid to be hard or soft? _____________
Answer: (a) strong; (b) high; (c) hard
![]() Ionic solids do not conduct electricity when solid, but when heated to the liquid state the molten ionic compounds conduct electricity well. In the liquid state, the crystal lattice is broken and the ions are free to move about and carry the electric current. Most ionic solids dissolve easily in water. The aqueous solutions of ionic solids also conduct electricity well.
Ionic solids do not conduct electricity when solid, but when heated to the liquid state the molten ionic compounds conduct electricity well. In the liquid state, the crystal lattice is broken and the ions are free to move about and carry the electric current. Most ionic solids dissolve easily in water. The aqueous solutions of ionic solids also conduct electricity well.
A very hard solid with a high melting point dissolves in water, but the aqueous solution does not conduct electricity. Would you expect the solid to be ionic?_________________
Answer: no (Ionic solids dissolve readily in water and conduct electricity.)
![]() Covalent solids (also known as network solids) consist of atoms held together completely by covalent bonds extending from atom to atom throughout the crystal lattice. The entire crystal lattice is interlocked in a series of such covalent bonds. Covalent bonds are very strong, and such solids are very hard with very high melting points. A relatively large amount of energy is required to break the bonds.
Covalent solids (also known as network solids) consist of atoms held together completely by covalent bonds extending from atom to atom throughout the crystal lattice. The entire crystal lattice is interlocked in a series of such covalent bonds. Covalent bonds are very strong, and such solids are very hard with very high melting points. A relatively large amount of energy is required to break the bonds.
A solid substance with a high melting point could be (an ionic solid, a covalent solid, either an ionic solid or a covalent solid) _______________
Answer: either an ionic solid or a covalent solid (Covalent solids often have higher melting points than ionic solids, but both have relatively high melting points.)
![]() Covalent solids are nonconductors of electricity in either the solid or the molten (liquid) state. Most covalent solids are not soluble in water. Typical examples of covalent solids are diamond (made of carbon), silicon carbide (SiC, better known as carborundum), and aluminum nitride (AlN). All of these examples are useful as industrial abrasives for grinding and polishing because of their hardness.
Covalent solids are nonconductors of electricity in either the solid or the molten (liquid) state. Most covalent solids are not soluble in water. Typical examples of covalent solids are diamond (made of carbon), silicon carbide (SiC, better known as carborundum), and aluminum nitride (AlN). All of these examples are useful as industrial abrasives for grinding and polishing because of their hardness.
A solid is heated until it melts at a high melting point. The molten substance conducts electricity. This solid is likely to be (an ionic solid, a covalent solid, either an ionic or covalent solid) _____________________
Answer: an ionic solid (Ionic solids conduct electricity when molten, while covalent solids do not.)
![]() Molecular solids are made up of either polar or nonpolar molecules. (See Chapter 3 if you need to review polar and nonpolar compounds.) Nonpolar molecules are held together by weak van der Waals forces. Note that the atoms within a molecule may be covalently bonded, but in a molecular solid the molecules themselves are not interconnected with covalent bonds. Both ionic and covalent solids are relatively hard and have high melting points because of the strong bonds within the solids. Try some predictions about the hardness and the melting points of nonpolar molecular solids. Would you expect nonpolar molecular solids with their weak interconnecting forces to:
Molecular solids are made up of either polar or nonpolar molecules. (See Chapter 3 if you need to review polar and nonpolar compounds.) Nonpolar molecules are held together by weak van der Waals forces. Note that the atoms within a molecule may be covalently bonded, but in a molecular solid the molecules themselves are not interconnected with covalent bonds. Both ionic and covalent solids are relatively hard and have high melting points because of the strong bonds within the solids. Try some predictions about the hardness and the melting points of nonpolar molecular solids. Would you expect nonpolar molecular solids with their weak interconnecting forces to:
1. be relatively hard or relatively soft? _________________
2. have melting points that are high or low? _______________
Answer: (a) relatively soft; (b) low melting points
![]() Two examples of nonpolar molecular solids are dry ice (CO2) and naphthalene (mothballs). Polar molecular solids such as ice (H2O), solid ammonia (NH3), and solid sulfuric acid (H2SO4) have melting points and degrees of hardness between those of nonpolar molecular solids and ionic solids. There is some electrostatic attraction between polar molecules. As a group, polar and nonpolar molecular solids are generally soft, have comparatively low melting points, and do not conduct electricity.
Two examples of nonpolar molecular solids are dry ice (CO2) and naphthalene (mothballs). Polar molecular solids such as ice (H2O), solid ammonia (NH3), and solid sulfuric acid (H2SO4) have melting points and degrees of hardness between those of nonpolar molecular solids and ionic solids. There is some electrostatic attraction between polar molecules. As a group, polar and nonpolar molecular solids are generally soft, have comparatively low melting points, and do not conduct electricity.
A solid is very hard but does not conduct electricity in the solid or liquid state. The solid is probably (molecular, ionic, covalent) _________________
Answer: covalent
![]() A fourth category of crystalline solids is metallic solids. Examples are elemental iron, copper, and silver. Solid metals have been described as made up of positive ions held together by highly mobile electrons or as positive ions in a “sea” of electrons. These electrons move freely, with the result that the metals are excellent conductors of electricity. The melting points and hardnesses of various metals vary over wide ranges. Which of the four types of crystalline solids conducts electricity as a solid: ionic, covalent, molecular, or metallic? __________
A fourth category of crystalline solids is metallic solids. Examples are elemental iron, copper, and silver. Solid metals have been described as made up of positive ions held together by highly mobile electrons or as positive ions in a “sea” of electrons. These electrons move freely, with the result that the metals are excellent conductors of electricity. The melting points and hardnesses of various metals vary over wide ranges. Which of the four types of crystalline solids conducts electricity as a solid: ionic, covalent, molecular, or metallic? __________
Answer: metallic (Ionic solids must be melted or dissolved before they will conduct electricity.)
![]() In metallic solids, each metal atom is surrounded by eight other atoms as its nearest neighbors in a cubic closest-packed crystalline structure, or 12 other atoms in a hexagonal closest-packed structure. The outer shell electrons are loosely held in each atom. The mobility of these electrons accounts for the fact that metals are excellent conductors of both electricity and heat.
In metallic solids, each metal atom is surrounded by eight other atoms as its nearest neighbors in a cubic closest-packed crystalline structure, or 12 other atoms in a hexagonal closest-packed structure. The outer shell electrons are loosely held in each atom. The mobility of these electrons accounts for the fact that metals are excellent conductors of both electricity and heat.
Metallic solids generally assume two closest-packed crystalline structures. What are these geometric structures? __________
Answer: cubic and hexagonal
![]() As you should recall, metallic solids also have certain characteristics such as malleability (can be rolled or beaten into fine sheets), ductility (can be drawn into fine wires), and luster (shine) in both the solid and molten states. Which two of the following characteristics of metallic solids best explain the use of some metallic solids in electrical wiring? (luster, malleability, ductility, cubic or hexagonal closest-packed crystal structure, mobility of outer shell electrons) ___________________________________________________
As you should recall, metallic solids also have certain characteristics such as malleability (can be rolled or beaten into fine sheets), ductility (can be drawn into fine wires), and luster (shine) in both the solid and molten states. Which two of the following characteristics of metallic solids best explain the use of some metallic solids in electrical wiring? (luster, malleability, ductility, cubic or hexagonal closest-packed crystal structure, mobility of outer shell electrons) ___________________________________________________
Answer: ductility and mobility of outer shell electrons
![]() You have just learned the properties of these types of solids: ionic, covalent, molecular, and metallic.
You have just learned the properties of these types of solids: ionic, covalent, molecular, and metallic.
1. Which are the hardest, so that some examples of these solids are used for industrial drilling and abrasives? __________
2. Which solids are the softest? __________
3. Which are the best conductors of electricity and heat? __________
4. Which solids are held in a crystalline structure by strong electrostatic attractions?______________
Answer: (a) covalent; (b) molecular; (c) metallic; (d) ionic
In the next section we will discuss how X-ray diffraction has been used to determine the sizes of atoms and ions and how the sizes vary from group to group and period to period in the periodic table.
ATOMIC AND IONIC RADII
![]() An important use of X-ray diffraction analysis is the determination of atomic and ionic sizes. Atomic and ionic sizes are usually listed in angstrom units, Å (1 Ångstrom unit = 1 × 10−8 centimeters). The radii of some typical atoms and their ions are listed below.
An important use of X-ray diffraction analysis is the determination of atomic and ionic sizes. Atomic and ionic sizes are usually listed in angstrom units, Å (1 Ångstrom unit = 1 × 10−8 centimeters). The radii of some typical atoms and their ions are listed below.
|
Sizes of Positive Ions and Their Parent Atoms |
|||
Atom |
Radius (Å) |
Ion |
Radius (Å) |
Li |
1.23 |
Li+ |
0.60 |
Na |
1.57 |
Na+ |
0.95 |
K |
2.03 |
K+ |
1.33 |
Rb |
2.16 |
Rb+ |
1.48 |
Cs |
2.35 |
Cs+ |
1.69 |
Be |
0.89 |
Be2+ |
0.31 |
Mg |
1.36 |
Mg2+ |
0.65 |
Ca |
1.74 |
Ca2+ |
0.99 |
Sr |
1.91 |
Sr2+ |
1.13 |
Ba |
1.98 |
Ba2+ |
1.35 |
In this chart, locate the Na atom. What is the atomic radius of a Na atom? ___________________
Answer: 1.57 Å (Remember to express atomic radii in angstrom units, Å.)
![]() Using the same chart, find the radius of the sodium ion, Na+. ______________
Using the same chart, find the radius of the sodium ion, Na+. ______________
Answer: 0.95 Å
![]() The positive ion Na+ has a smaller radius than its parent atom Na. Look at all the positive ions listed on the chart and compare the positive ions to their parent atoms. How do the radii of positive ions compare with the radii of their parent atoms? ________________________________________________________
The positive ion Na+ has a smaller radius than its parent atom Na. Look at all the positive ions listed on the chart and compare the positive ions to their parent atoms. How do the radii of positive ions compare with the radii of their parent atoms? ________________________________________________________
Answer: Positive ions are all smaller than their parent atoms.
![]() Positive ions are smaller than their parent atoms because a positive ion has lost one or more electrons. The protons now outnumber the electrons, so the protons (which are in the nucleus) exhibit more attraction for the remaining electrons. The protons thus draw the remaining electrons closer to the nucleus, resulting in a smaller radius than the original atom. In addition, electrons are lost from the outermost shell. Loss of these outermost shell electrons also makes the ion smaller than the original atom.
Positive ions are smaller than their parent atoms because a positive ion has lost one or more electrons. The protons now outnumber the electrons, so the protons (which are in the nucleus) exhibit more attraction for the remaining electrons. The protons thus draw the remaining electrons closer to the nucleus, resulting in a smaller radius than the original atom. In addition, electrons are lost from the outermost shell. Loss of these outermost shell electrons also makes the ion smaller than the original atom.
A negative ion has gained one or more electrons. Based upon what you have just learned about positive ions, how should the radius of a negative ion compare with the radius of its parent atom? ___________________________________
Answer: The negative ion radius should be larger than the radius of the parent atom.
|
Sizes of Negative Ions and Their Parent Atoms |
|||
Atom |
Radius (Å) |
Ion |
Radius (Å) |
F |
0.72 |
F— |
1.36 |
Cl |
0.99 |
Cl— |
1.81 |
Br |
1.14 |
Br— |
1.95 |
I |
1.33 |
I— |
2.16 |
O |
0.74 |
O2— |
1.40 |
S |
1.04 |
S2— |
1.84 |
Se |
1.17 |
Se2— |
1.98 |
Te |
1.37 |
Te2— |
2.21 |
![]() Look at the chart of sizes of negative ions and their parent atoms.
Look at the chart of sizes of negative ions and their parent atoms.
1. What is the radius of an O2— ion? __________
2. How many centimeters is it? __________
Answer:
1. 1.40 Å
2. 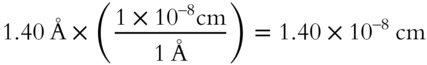
![]() Ions and atoms that have the same number of electrons are called isoelectronic. The ion Na+ has the same number of electrons as an atom of Ne. All of the following have the same number of electrons and are isoelectronic:
Ions and atoms that have the same number of electrons are called isoelectronic. The ion Na+ has the same number of electrons as an atom of Ne. All of the following have the same number of electrons and are isoelectronic:
![]()
Do any two of these isoelectronic ions or atoms have the same number of protons? ________________________
Answer: no (Remember that only the number of electrons is changed. The number of protons in the nucleus remains unchanged and is a fixed number for each element, its atomic number.)
![]() An increase in the positive charge per electron results in a decrease in the radius. All of the following have 10 electrons, so they are isoelectronic. Their atomic numbers (shown below the isoelectronic ions) range from 7 to 14.
An increase in the positive charge per electron results in a decrease in the radius. All of the following have 10 electrons, so they are isoelectronic. Their atomic numbers (shown below the isoelectronic ions) range from 7 to 14.
N3— |
O2— |
F— |
Ne |
Na+ |
Mg2+` |
Al3+ |
Si4+ |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
In the negative ions, the electrons outnumber the protons. In the positive ions, the protons outnumber the electrons. Would you expect the radii of these isoelectronic species to increase from left to right or to decrease from left to right? ______________________________
Answer: decrease from left to right (The number of protons increases while the number of electrons remains constant, therefore increasing the attraction on the electrons and making each successive ion smaller.)
When an atom has more protons than electrons, the increase in the positive charge per electron results in a decrease in the radius. When an atom has fewer protons than electrons, the decrease in the positive charge per electron results in an increase in the radius.
Iron, Fe, has an atomic number of 26. A neutral atom of Fe has 26 electrons. An Fe2+ ion has 24 electrons. An Fe3+ ion has 23 electrons. List the Fe atom and its two ions in order of decreasing size (largest first). __________________
Answer: Fe, Fe2+, Fe3+
You have just learned some of the properties of solids and how they compare with gases. Our knowledge of the structures of solids and their properties helps us to develop new uses for solids and new combinations of solids that are stronger, better conductors of heat and electricity, and more resistant to chemical attack.
In Chapter 10 we will discuss the liquid state and its properties. A comparison of liquids to the solid and gaseous states is also presented.
SELF-TEST
This self-test is designed to show how well you have mastered this chapter's objectives. Correct answers and review instructions follow the test.
1. The temperature at which a solid changes to a liquid is which, its melting point or freezing point?
2. Dry ice, the solid form of carbon dioxide gas, does not melt to liquid carbon dioxide. Instead solid CO2 directly changes to gaseous CO2. The process of changing from a solid directly to a gas is known as ________.
3. There are _____ known basic crystal systems.
4. The process known as ___________ allows us to determine the arrangement of atoms, ions, and molecules within a crystal lattice.
5. Sulfur exists in more than one crystalline form. Is it amorphous or polymorphic? ________________________________
6. Graphite and diamond are two forms of carbon. What are they called? (amorphous, allotropes, isotopes) ____________________________
7. State at least two basic differences between crystals and glasses. _______________________________________________________
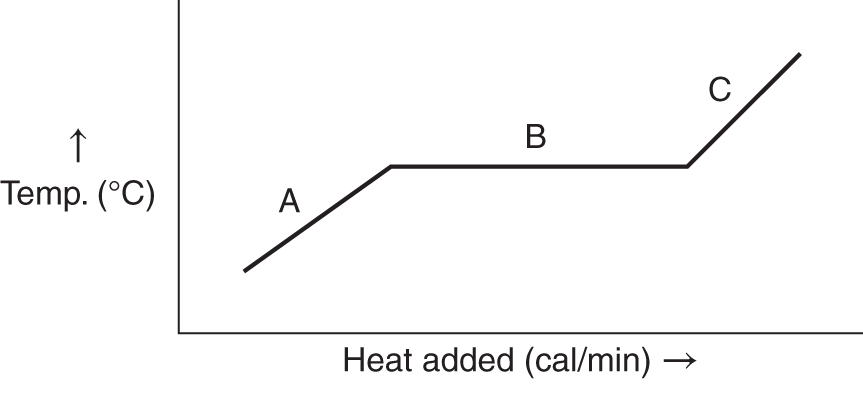
8. The graph below represents the heating of a solid. Indicate what is happening or what is represented by each of the labeled points.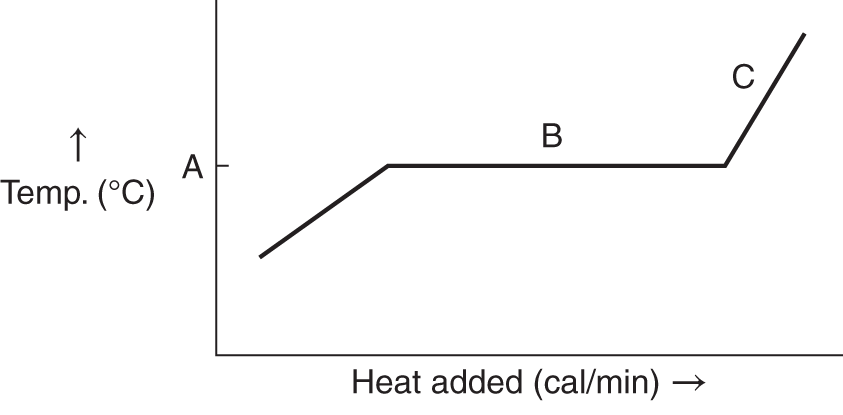
A _________________________________________________________
B _________________________________________________________
C _________________________________________________________
9. The heat required to raise the temperature of 1 gram of substance by 1oC is called the _______ of a substance.
10. What is the approximate atomic weight (to the nearest tenth) of aluminum if it has a specific heat of 0.215 cal/g?
11. What is the approximate atomic weight (to the nearest tenth) of titanium if it has a specific heat of 0.125 cal/g?
12. Which one of each of the following sets will have the larger radius?
1. Mg or Mg2+__________
2. S or S2—__________
3. F— or Ne__________
4. Ca2+, K+, F—, or O2— __________
13. Which one of each of the following sets will have the smaller radius?
1. Ca or Ca2+ _____________
2. P or P3— _______________
3. Li+ or Br— _____________
4. F—, Cl—, Br—, or I— ________
14. Which of the following ions and atoms are isoelectronic with argon (Ar)? (Cl−, S2−, P, K, Ca2+, Kr) ________________________________
15. Which of the following are isoelectronic with krypton (Kr)? (Cl—, Br—, Rb+, As3—, Se, K+)
ANSWERS
Compare your answers to the self-test with those given below. If you answer all questions correctly, you are ready to proceed to the next chapter. If you miss any, review the frames indicated in parentheses following the answers. If you miss several questions, you should probably reread the chapter carefully.
1. for many substances the melting and freezing point temperature is the same with both solid and liquid present at the same time but since the question noted “solid to liquid”, the best answer is melting point [frames 1-3]
2. sublimation [frame 4]
3. seven [frame 5]
4. X-ray diffraction [frame 5]
5. polymorphic (frame 6)
6. allotropes (frame 7)
7.
1. Crystals have sharp melting points, while glasses have broad melting point ranges.
2. Crystals cleave along planar surfaces, while glasses fracture to form rounded or uneven surfaces.
3. Crystals have equal bond strengths in all directions, while glasses have variable bond strengths.
4. Crystals diffract X-rays, but glasses do not. (frames 9—12)
8.
1. melting point
2. heat of fusion (solid and liquid present while the solid is melting)
3. only liquid present, while temperature is increasing (frames 15—20)
9. specific heat [frame 22]
10.
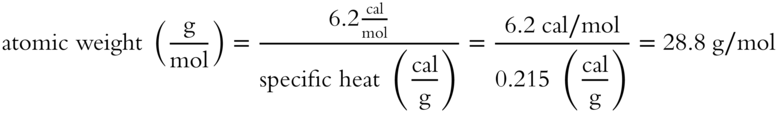 Due to this being an approximation it is relatively close to the atomic weight of aluminum which is 26.982 g/mol. [frames 21-26]]
Due to this being an approximation it is relatively close to the atomic weight of aluminum which is 26.982 g/mol. [frames 21-26]]
11.
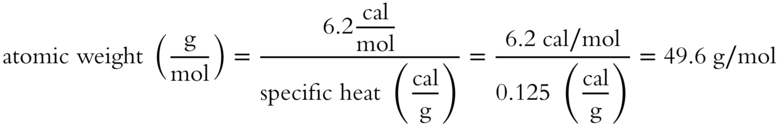 Due to this being an approximation it is relatively close to the atomic weight of titanium which is 47.867 g/mol. [frames 21-26]]
Due to this being an approximation it is relatively close to the atomic weight of titanium which is 47.867 g/mol. [frames 21-26]]
12. (a) Mg, (b) S2—, (c) F—, (d) O2— (frames 37—40, 43)
13. (a) Ca2+ (b) P (c) Li+ (d) F− [frames 37-40, 43]
14. Cl—, S2—, Ca2+ (frame 42)
15. Br—, Rb+, As3— [frame 42]
EVERYDAY CHEMISTRY
Piezoelectric Crystals
In this chapter, you learned about some solids that are crystals with very specific shapes. Did you know that some crystals when struck or deformed just a little can produce a spike of electricity? If you have ever used an outdoor gas grill, many have a red button that when pressed actuates a small spring hammer that strikes a crystal which produces a momentary high voltage that is conducted by wire to a gap, resulting in a spark. That spark ignites the gas and air mixture in the gas grill. No battery is needed and the process can be repeated many times as long as the mechanical and electrical connections are intact. A crystal of this type is piezoelectric. Not only is the crystal capable of producing a spike of electricity but the reverse can also be true. A very accurate quartz watch relies on the effect of a crystal that is excited by a tiny electric current vibrates at a consistent rate depending on how it is cut and its thickness. That same type of crystal is used in computers and many other devices to provide accurate timing.
A crystal can be carefully cut in shape and size to produce a vibration at a specific frequency when subjected to electric current. During World War II, the United States relied on Brazil to mine quartz crystals which were cut and shaped so that radio transmitters for communication could be relied on at various selected radio frequencies. The shortage of naturally occurring quartz for cutting those crystals caused a major research effort to produce man-made crystals. Most of the crystals in use today are man-made. The piezoelectric effect is also relied on for tiny speakers and beepers as well as for larger devices used in ultrasound.
The reason that a crystal can produce electricity when mechanically stressed and deformed slightly or can vibrate when subjected to an alternating electric current is that the arrangement of atoms and electrical charges within the crystal, called dipole moments, are balanced and therefore neutral. When squeezed, an unbalance occurs causing the electrical charges to no longer be neutral and producing a momentary voltage on two faces of a crystal until the crystal returns to its balanced position. Feeding an alternating electric current to the crystal faces causes the crystal to deform and vibrate.
The development of piezoelectric devices and materials is a fruitful area for continuing research in chemistry.