Chemistry: A Self-Teaching Guide - Post R., Snyder C., Houk C.C. 2020
Liquids
Liquids are easily recognized by comparison with the most widely known liquid, water. You know from experience that water flows when we pour it, boils when heated enough, freezes when cooled enough, disappears when left in the open, does not mix with oil or gasoline, supports “water bugs” on its surface, and cools when it evaporates, as when perspiration leaves your skin. These properties of water are characteristic of all liquids. Every liquid has its own boiling point, freezing point, flow, surface, and “heat” properties.
This chapter will acquaint you with the characteristics of the liquid state. Every gas becomes a liquid when it is cooled to a sufficiently low temperature. This is the result of intermolecular attractions. The properties of liquids are largely determined by the magnitude of these intermolecular forces. The forces most often encountered are van der Waals forces or hydrogen bonds, formed by polar molecules, and very weak forces called London forces, which are primarily of interest in the behavior of nonpolar molecules.
Every liquid becomes a solid when sufficiently cooled. This happens at the temperature at which the kinetic energy of the molecules becomes so small that intermolecular attraction brings about a complete loss of fluidity. The solid that results has a rigid structure that is characterized by its own unique type of crystal shape. Liquids fall between gases and solids in their “ordering.” Remember, gases fill their container and take its shape, and are in rapid random motion with no order.
Solids, on the other hand, are very highly ordered and do not fill or take the shape of their container unless forced to do so.
OBJECTIVES
After you have completed this chapter, you will be able to
· recognize and apply or illustrate: boiling point, vapor pressure, viscosity, surface tension, miscible, immiscible, volatility, density, hydrogen bonding, polar compounds, heat of vaporization, equilibrium, and Le Chatelier's Principle;
· state the effect of temperature on the surface tension, viscosity, and volatility of a liquid;
· give an explanation of why the vapor pressure of a liquid changes with temperature and how this relates to boiling point and critical temperature;
· explain what properties are necessary for a good refrigerant;
· given a graph representing what happens to the temperature of a substance as heat is added to it, locate the melting point, the boiling point, the states of matter that are present, and the regions representing heats of fusion and vaporization;
· given the graph of the vapor pressure versus the temperature of a liquid, determine: the vapor pressure of the liquid at a specific temperature and the boiling point of the liquid at a specific atmospheric pressure;
· calculate the amount of heat that must be supplied to vaporize a certain amount of a liquid when given its heat of vaporization;
· predict whether or not two liquids will mix when put together.
![]() We begin this chapter with a brief comparison of the states of matter based upon the kinetic molecular theory. The kinetic molecular theory was first encountered in explaining the behavior of gases. When cooled to a sufficiently low temperature, the average kinetic energy drops and every gas becomes a liquid. The molecules of liquid substances still move in random motion according to the kinetic molecular theory. However, the molecules in the liquid state move more slowly, and there is very little space between molecules.
We begin this chapter with a brief comparison of the states of matter based upon the kinetic molecular theory. The kinetic molecular theory was first encountered in explaining the behavior of gases. When cooled to a sufficiently low temperature, the average kinetic energy drops and every gas becomes a liquid. The molecules of liquid substances still move in random motion according to the kinetic molecular theory. However, the molecules in the liquid state move more slowly, and there is very little space between molecules.
When a gas is compressed, the space between the molecules of a gas is actually compressed. Since there is very little space between the molecules of a liquid, would you expect liquids to be readily compressed? _______
Answer: no (In reality, even large pressures have very little effect on the volume of a liquid.)
![]() The molecules of a liquid are not as structured as those in the solid state. The liquid molecules are free to slide over one another and to flow and assume the shape of the container. The molecules of a solid can vibrate and bend but cannot flow and slide over one another. The three states of matter (gaseous, liquid, and solid) differ in terms of the freedom of movement of molecules.
The molecules of a liquid are not as structured as those in the solid state. The liquid molecules are free to slide over one another and to flow and assume the shape of the container. The molecules of a solid can vibrate and bend but cannot flow and slide over one another. The three states of matter (gaseous, liquid, and solid) differ in terms of the freedom of movement of molecules.
1. Molecules have the greatest freedom of movement in which state?_______________________
2. Molecules have the least freedom of movement in which state?__________
Answer: (a) gaseous; (b) solid
![]() Many of the properties of liquids are determined by the attraction of molecules for each other. These intermolecular attractive forces cause the molecules to stick together and coalesce into a liquid. The intermolecular attractions affect the flow rate of a liquid. Heavy oils, syrup, and molasses flow more slowly than water or gasoline. Compared to liquids that flow rather quickly, liquids of slower flow rate would have (stronger, weaker) __________ intermolecular attractive forces.
Many of the properties of liquids are determined by the attraction of molecules for each other. These intermolecular attractive forces cause the molecules to stick together and coalesce into a liquid. The intermolecular attractions affect the flow rate of a liquid. Heavy oils, syrup, and molasses flow more slowly than water or gasoline. Compared to liquids that flow rather quickly, liquids of slower flow rate would have (stronger, weaker) __________ intermolecular attractive forces.
Answer: stronger (The stronger the intermolecular attraction, the more the molecules stick to each other and the slower the flow rate of a liquid.)
![]() The viscosity of a liquid refers to its resistance to flow. A liquid with a high resistance to flow such as heavy oils or molasses would exhibit high viscosity. A liquid with very little resistance to flow would exhibit low viscosity. High viscosity is associated with relatively (strong, weak) __________ intermolecular attractive forces.
The viscosity of a liquid refers to its resistance to flow. A liquid with a high resistance to flow such as heavy oils or molasses would exhibit high viscosity. A liquid with very little resistance to flow would exhibit low viscosity. High viscosity is associated with relatively (strong, weak) __________ intermolecular attractive forces.
Answer: strong
![]() A liquid with relatively weak intermolecular forces would probably exhibit low __________ (use the term that refers to resistance to flow).
A liquid with relatively weak intermolecular forces would probably exhibit low __________ (use the term that refers to resistance to flow).
Answer: viscosity
![]() Razor blades and certain insects that are heavier than water can float on top of the water due to surface tension. Remember that the molecules of a liquid attract each other through intermolecular attractive forces. Surface tension is caused by a difference in direction of intermolecular attractive forces between those molecules at the surface of a liquid and those in the body of the liquid.
Razor blades and certain insects that are heavier than water can float on top of the water due to surface tension. Remember that the molecules of a liquid attract each other through intermolecular attractive forces. Surface tension is caused by a difference in direction of intermolecular attractive forces between those molecules at the surface of a liquid and those in the body of the liquid.
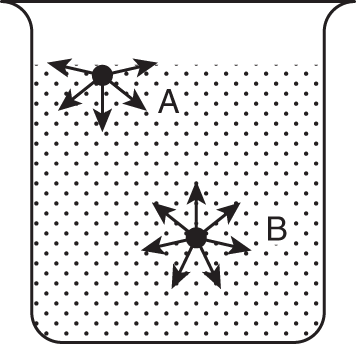
A molecule in the body of the liquid (molecule B) is surrounded by attraction from other liquid molecules. There is equal attraction from all sides. A molecule at the surface of the liquid (molecule A) is attracted only toward the interior of the liquid. It is not balanced by an attraction from above. Which molecule (A or B) is involved in surface tension? _________________
Answer: molecule A
![]() Because molecule A and all other molecules making up the surface of the liquid are pulled inward by intermolecular attraction, the liquid tends to form as small a surface as possible. This inward pull of the surface molecules is known as surface tension.
Because molecule A and all other molecules making up the surface of the liquid are pulled inward by intermolecular attraction, the liquid tends to form as small a surface as possible. This inward pull of the surface molecules is known as surface tension.
A sphere is the three-dimensional figure with the smallest surface area. Water and other liquids tend to form droplets or small spheres because the inward pull on the surface molecules tends to produce as small a surface area as possible. A falling raindrop is actually a small sphere that is distended somewhat by the pull of gravity.
Liquids tend to form spherical droplets because of the inward pull on surface molecules, which is called __________________
Answer: surface tension
![]() The inward pull of surface molecules depends upon intermolecular attractive forces. Strong intermolecular attractive forces provide a strong inward pull. Compared to a liquid with relatively weak intermolecular attractive forces, a liquid with relatively strong intermolecular forces could be expected to show:
The inward pull of surface molecules depends upon intermolecular attractive forces. Strong intermolecular attractive forces provide a strong inward pull. Compared to a liquid with relatively weak intermolecular attractive forces, a liquid with relatively strong intermolecular forces could be expected to show:
1. (greater, lesser) _______________ surface tension
2. (higher, lower) ________________ viscosity
Answer: (a) greater; (b) higher
VAPOR PRESSURE
![]() Vapor pressure is the pressure exerted by the vapor (gaseous) phase of a liquid in a closed container. To understand vapor pressure, you must understand several other concepts. You may already be familiar with evaporation. Evaporation means passing from the liquid state to the gaseous (vapor) state. Remember that all of the molecules in a liquid together have an average kinetic energy that is a measure of the temperature of the liquid. However, not all individual molecules have the same kinetic energy. Some have above average and some have below average kinetic energy. Some molecules with greater kinetic energy will escape from the liquid if they are located near the surface. These molecules evaporate and enter the gaseous (vapor) state.
Vapor pressure is the pressure exerted by the vapor (gaseous) phase of a liquid in a closed container. To understand vapor pressure, you must understand several other concepts. You may already be familiar with evaporation. Evaporation means passing from the liquid state to the gaseous (vapor) state. Remember that all of the molecules in a liquid together have an average kinetic energy that is a measure of the temperature of the liquid. However, not all individual molecules have the same kinetic energy. Some have above average and some have below average kinetic energy. Some molecules with greater kinetic energy will escape from the liquid if they are located near the surface. These molecules evaporate and enter the gaseous (vapor) state.
1. The molecules of liquid that evaporate first are those of (higher, lower) ________________ than average kinetic energy.
2. If a saucer of water is uncovered and not touched for a day or two, some of the water “disappears.” In terms of the kinetic molecular theory, explain what happened to the water that is missing. ________________
Answer: (a) higher; (b) Some of the water has evaporated. Those molecules with greater than average kinetic energy that are located near the surface of the liquid have escaped and entered the vapor state.
![]() The molecules of greatest kinetic energy evaporate first, while the molecules of lower kinetic energy remain. The result is a drop in temperature of the remaining molecules. (Temperature is simply a measurement of the average kinetic energy of the molecules. As the average kinetic energy drops, so does the temperature.)
The molecules of greatest kinetic energy evaporate first, while the molecules of lower kinetic energy remain. The result is a drop in temperature of the remaining molecules. (Temperature is simply a measurement of the average kinetic energy of the molecules. As the average kinetic energy drops, so does the temperature.)
A liquid in a well-insulated container has a rapid rate of evaporation. Its temperature is measured prior to evaporation. After evaporation of a portion of the liquid, would you expect the temperature of the remaining liquid to be higher or lower than the original temperature? ________________
Answer: lower (because the average kinetic energy of the remaining liquid molecules is also lower)
![]() To make up for the loss of kinetic energy through evaporation, a liquid undergoing evaporation will draw heat from its surroundings. That is why rubbing alcohol feels cold on your skin as it evaporates. Heat is removed from the skin during evaporation. Sweating is a means used by your body to control its temperature. A natural breeze or the breeze from a fan can increase the rate of evaporation. As a result, your skin temperature is (warmed, cooled) ________________.
To make up for the loss of kinetic energy through evaporation, a liquid undergoing evaporation will draw heat from its surroundings. That is why rubbing alcohol feels cold on your skin as it evaporates. Heat is removed from the skin during evaporation. Sweating is a means used by your body to control its temperature. A natural breeze or the breeze from a fan can increase the rate of evaporation. As a result, your skin temperature is (warmed, cooled) ________________.
Answer: cooled (The evaporating sweat draws away heat and cools the skin.)
![]() If a container of liquid has a cover, the liquid will continue to evaporate, but after a time, some of the vapor (gaseous) molecules will return to the liquid state in a process called condensation, which is essentially the opposite of evaporation. After a longer time, the rate of condensation will equal the rate of evaporation. When an equal rate of evaporation and condensation is reached, the pressure exerted by the vapor molecules is known as the vapor pressure.
If a container of liquid has a cover, the liquid will continue to evaporate, but after a time, some of the vapor (gaseous) molecules will return to the liquid state in a process called condensation, which is essentially the opposite of evaporation. After a longer time, the rate of condensation will equal the rate of evaporation. When an equal rate of evaporation and condensation is reached, the pressure exerted by the vapor molecules is known as the vapor pressure.
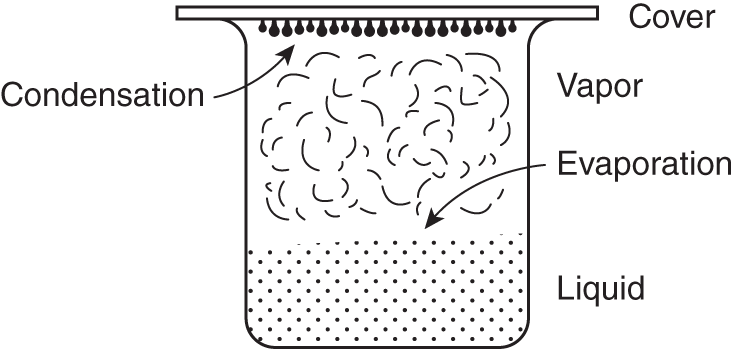
When the rate of evaporation is equal to the rate of condensation, the liquid and vapor are said to be in equilibrium. Liquid molecules continue to evaporate when equilibrium is reached, but just as many vaporized molecules condense into liquid. Both processes continue at equal rates. The processes are opposite but equal in rate.

In order for equilibrium to be maintained with two opposite processes, the rates of the two opposite processes must be _____________.
Answer: equal
![]() An increase in temperature will increase the rate of evaporation so that the system is no longer in equilibrium. The system will achieve a new equilibrium at the new temperature, however, because the rate of condensation also increases until both rates are again equal.
An increase in temperature will increase the rate of evaporation so that the system is no longer in equilibrium. The system will achieve a new equilibrium at the new temperature, however, because the rate of condensation also increases until both rates are again equal.
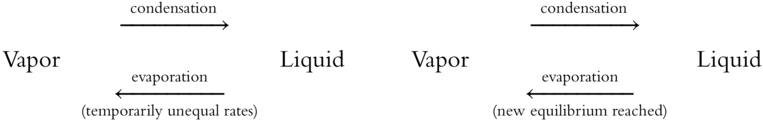
An increase in the confining pressure will result in an increase in the rate of condensation. What could be expected to happen to the two rates after a period of time at the new pressure? ________________
Answer: After a period of time, a new equilibrium is formed and the rates of evaporation and condensation would again be equal.
![]() A change in temperature or pressure will cause a change in the rates of evaporation or condensation. After a time, a new equilibrium is set up, with the result that the rates of evaporation and condensation are again equal, although the rates may be somewhat faster or slower than in the previous equilibrium. Le Chatelier's Principle governs equilibrium systems. According to Le Chatelier's Principle, a “stress” on a system in equilibrium will cause a shift to a new equilibrium so as to relieve the stress.
A change in temperature or pressure will cause a change in the rates of evaporation or condensation. After a time, a new equilibrium is set up, with the result that the rates of evaporation and condensation are again equal, although the rates may be somewhat faster or slower than in the previous equilibrium. Le Chatelier's Principle governs equilibrium systems. According to Le Chatelier's Principle, a “stress” on a system in equilibrium will cause a shift to a new equilibrium so as to relieve the stress.
The “stress” placed on the equilibrium system of evaporation and condensation rates can be a change in either ________________ or ________________
Answer: temperature; pressure
![]() Le Chatelier's Principle governs the direction of change in a system that is at equilibrium and then undergoes a stress to attain a new equilibrium. An increase in temperature in a vapor-liquid equilibrium causes an increase in the evaporation rate. To achieve equilibrium, how must the condensation rate change? ________________________________________________
Le Chatelier's Principle governs the direction of change in a system that is at equilibrium and then undergoes a stress to attain a new equilibrium. An increase in temperature in a vapor-liquid equilibrium causes an increase in the evaporation rate. To achieve equilibrium, how must the condensation rate change? ________________________________________________
Answer: The condensation rate must also increase.
![]() At the new equilibrium, both evaporation and condensation rates are again equal, although both rates are increased when compared to the previous equilibrium. Remember that vapor pressure is the pressure that a vapor exerts when a liquid and its vapor (evaporation and condensation) are at equilibrium (at a specified temperature). According to Le Chatelier's Principle, an increase in temperature can shift the equilibrium to increase the rates of evaporation and condensation to form a new equilibrium. The average kinetic energy of the liquid molecules is also increased according to the temperature increase. Similarly, a decrease in temperature can shift the equilibrium to decrease the rates of evaporation and condensation (and decrease the average kinetic energy). The greater the rate of evaporation, the greater the vapor pressure.
At the new equilibrium, both evaporation and condensation rates are again equal, although both rates are increased when compared to the previous equilibrium. Remember that vapor pressure is the pressure that a vapor exerts when a liquid and its vapor (evaporation and condensation) are at equilibrium (at a specified temperature). According to Le Chatelier's Principle, an increase in temperature can shift the equilibrium to increase the rates of evaporation and condensation to form a new equilibrium. The average kinetic energy of the liquid molecules is also increased according to the temperature increase. Similarly, a decrease in temperature can shift the equilibrium to decrease the rates of evaporation and condensation (and decrease the average kinetic energy). The greater the rate of evaporation, the greater the vapor pressure.
Would you expect the vapor pressure of a liquid to increase or decrease if the temperature is increased? ________________
Answer: increase (An increase in average kinetic energy allows more higher energy molecules to evaporate. The increased evaporation rate would increase the amount of vapor and the pressure it exerts.)
BOILING POINT
![]() Water is the most widely known liquid. We have seen water freeze, flow when poured, or boil when the temperature is high enough. The boiling point of water is usually listed as 100°C. This is true only if the atmospheric pressure is 1 atmosphere (1 atm, which equals 760 torr or 101.325 kilopascals, kPa). At higher pressure, the boiling point of water is raised. At pressures lower than 1 atm, the boiling point of water is below 100°C.
Water is the most widely known liquid. We have seen water freeze, flow when poured, or boil when the temperature is high enough. The boiling point of water is usually listed as 100°C. This is true only if the atmospheric pressure is 1 atmosphere (1 atm, which equals 760 torr or 101.325 kilopascals, kPa). At higher pressure, the boiling point of water is raised. At pressures lower than 1 atm, the boiling point of water is below 100°C.
When water is boiled in a pressure cooker, the pressure inside is greater than 1 atm. The boiling point of water could be expected to be (100°C, below 100°C, above 100°C) ________________.
Answer: above 100°C
![]() The boiling point of a liquid is the temperature at which the vapor pressure of a liquid equals the surrounding pressure. Based upon this definition, water would boil at 100°C at 1 atmosphere pressure because its vapor pressure was also equal to _____________ at that temperature.
The boiling point of a liquid is the temperature at which the vapor pressure of a liquid equals the surrounding pressure. Based upon this definition, water would boil at 100°C at 1 atmosphere pressure because its vapor pressure was also equal to _____________ at that temperature.
Answer: 1 atm
![]() The surrounding pressure of an open pan of boiling water is usually 1 atm. In the pressure cooker, the surrounding pressure is greater than 1 atm. In order for water to boil in a pressure cooker, the vapor pressure must equal this greater surrounding pressure.
The surrounding pressure of an open pan of boiling water is usually 1 atm. In the pressure cooker, the surrounding pressure is greater than 1 atm. In order for water to boil in a pressure cooker, the vapor pressure must equal this greater surrounding pressure.
Suppose that instead of increasing the surrounding pressure as with a pressure cooker, we attempted to boil water at a pressure of less than 1 atm, for example, on a high mountain. Would you expect water to boil at 100°C, below 100°C, or above 100°C? ________________
Answer: below 100°C (In fact, the boiling point of water on a high mountain, where the atmospheric pressure is less than 1 atm, is below 100°C.)
![]() The higher the temperature, the greater the vapor pressure. When the vapor pressure of a liquid equals the surrounding pressure (pressure of the outside atmosphere or in some vessel such as a pressure cooker), the liquid will boil. The graph below plots the vapor pressure of water against temperature.
The higher the temperature, the greater the vapor pressure. When the vapor pressure of a liquid equals the surrounding pressure (pressure of the outside atmosphere or in some vessel such as a pressure cooker), the liquid will boil. The graph below plots the vapor pressure of water against temperature.
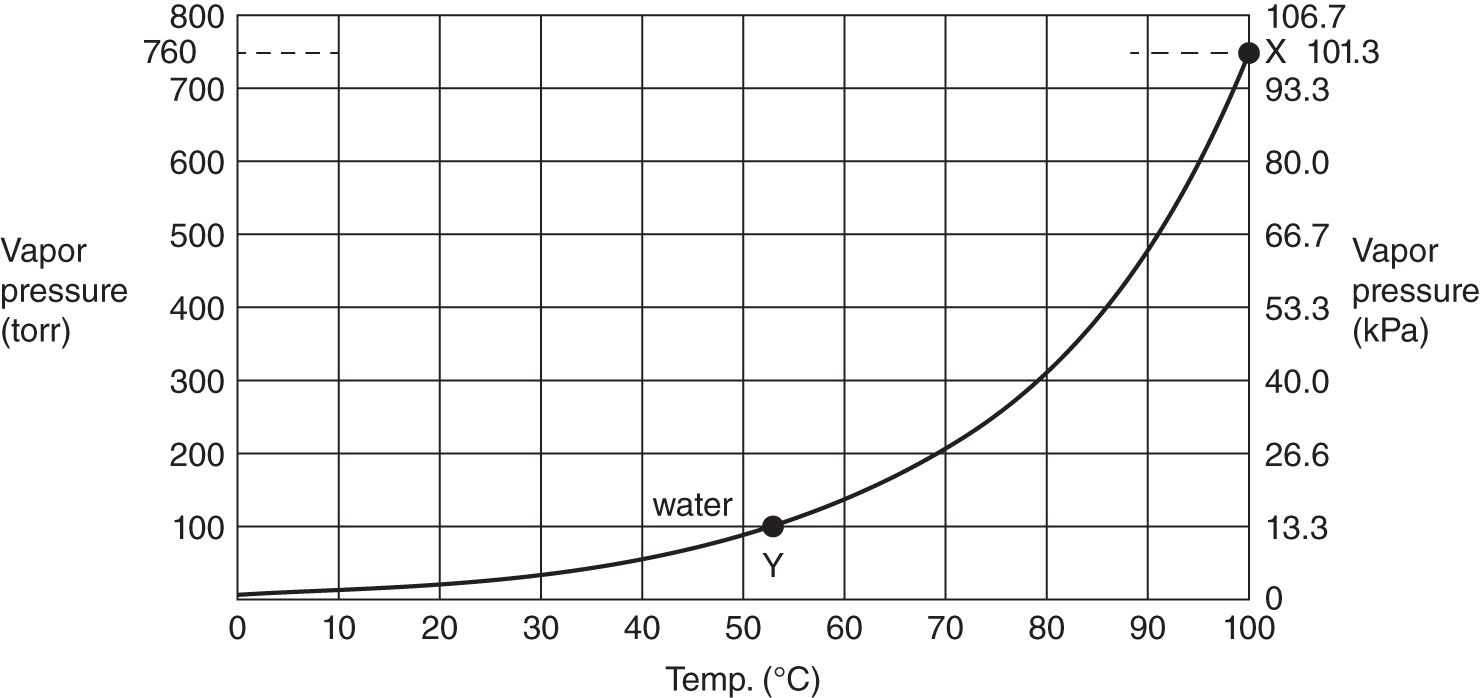 Change of vapor pressure with temperature for water
Change of vapor pressure with temperature for water
1. At the normal boiling point of water (100°C), what is its vapor pressure? (See point X on the graph.) ________________
2. Water can be made to boil at various temperatures, provided that the vapor pressure is equal to the surrounding or confining pressure. If the surrounding pressure is only 350 torr (46.7 kPa), at what vapor pressure would the water boil? ________________
3. Using the graph, at what temperature (approximate) would water boil if the surrounding pressure was only 100 torr (13.3 kPa)? (See point Y on the graph.) ______________________
Answer: (a) 760 torr or 1 atm or 101.3 kPa; (b) 350 torr or 46.7 kPa (remember that, at boiling, the vapor pressure of the liquid equals the surrounding pressure); (c) slightly more than 50°C (At that temperature, the vapor pressure is 100 torr or 13.3 kPa. If the surrounding pressure and vapor pressure are equal, water boils.)
![]() The question of the boiling points of other liquids may have occurred to you. Do all liquids have the same boiling points? Look at the graph on the following page.
The question of the boiling points of other liquids may have occurred to you. Do all liquids have the same boiling points? Look at the graph on the following page.
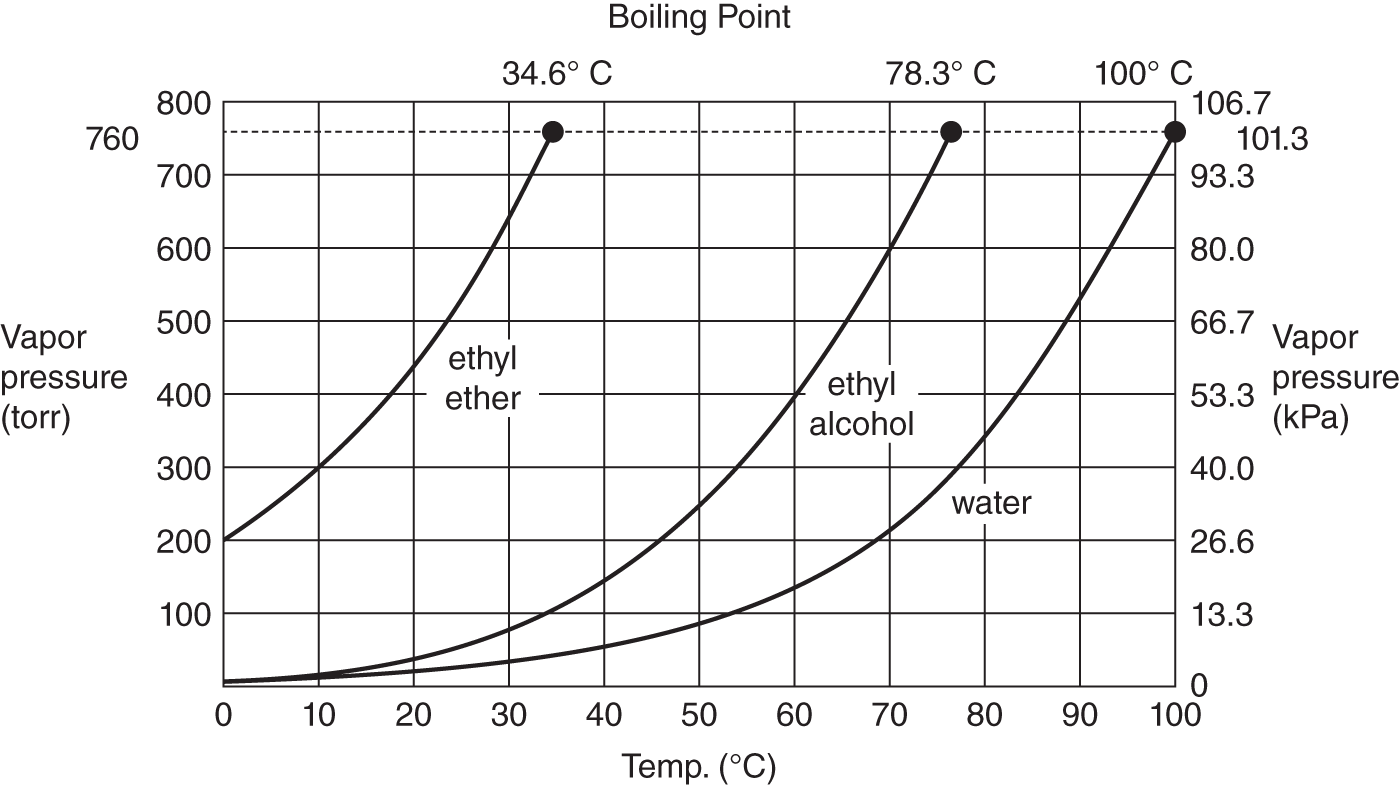
Vapor pressure and temperature for three common liquids
At a standard pressure of 1 atm (760 torr, 101.3 kPa), what is the boiling point of:
1. water? __________________________
2. ethyl alcohol? ________________
3. ethyl ether? ________________
Answer: (a) 100°C; (b) 78.3°C; (c) 34.6°C
![]() As a general rule, compounds of elements in the same family on the periodic table increase in boiling point with increasing molecular weight if all other factors are equal.
As a general rule, compounds of elements in the same family on the periodic table increase in boiling point with increasing molecular weight if all other factors are equal.
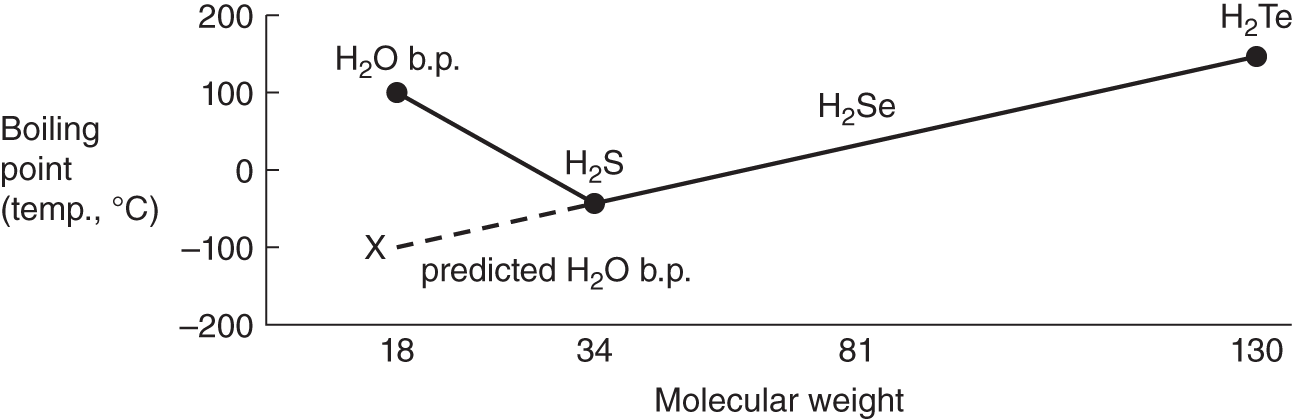
However, all factors are not always equal. Water (H2O), for example, has a predicted boiling point of below 0°C when only molecular weights are considered. Another factor must account for the much higher than predicted boiling point of water.
1. What is the approximate boiling point of water as predicted by the graph above? (See point X on the graph.) ______________
2. What is the actual boiling point of water at 1 atm? ________________
Answer: (a) −100°C (approximately); (b) 100°C
![]() The boiling point of a liquid is affected by the strength of the intermolecular attractive forces. Several kinds of forces can be classified as intermolecular attractions. One type of intermolecular attractive force is caused by polar molecules (first encountered in Chapter 3). Polar molecules have small electrostatic charges on opposite ends of the molecules. As a result, the molecules attract each other. There are also other types of intermolecular attractive forces such as van der Waals forces and London forces. You have already encountered van der Waals forces. London forces are much weaker intermolecular attractions in nonpolar compounds. They are caused by electrons from one molecule affecting the orbit of electrons in another molecule at very specific and constantly changing moments in time. Polar compounds have much stronger intermolecular attractions than nonpolar compounds. Stronger intermolecular attractions tend to keep molecules within the liquid form, with the result that the boiling point is higher than that predicted by molecular weight comparisons.
The boiling point of a liquid is affected by the strength of the intermolecular attractive forces. Several kinds of forces can be classified as intermolecular attractions. One type of intermolecular attractive force is caused by polar molecules (first encountered in Chapter 3). Polar molecules have small electrostatic charges on opposite ends of the molecules. As a result, the molecules attract each other. There are also other types of intermolecular attractive forces such as van der Waals forces and London forces. You have already encountered van der Waals forces. London forces are much weaker intermolecular attractions in nonpolar compounds. They are caused by electrons from one molecule affecting the orbit of electrons in another molecule at very specific and constantly changing moments in time. Polar compounds have much stronger intermolecular attractions than nonpolar compounds. Stronger intermolecular attractions tend to keep molecules within the liquid form, with the result that the boiling point is higher than that predicted by molecular weight comparisons.
Compound A and compound B have approximately the same molecular weight. However, compound A has a much higher boiling point than compound B. Based upon what you have just learned, which compound (A or B) could be expected to have the stronger intermolecular attractive forces? ________________
Answer: compound A
![]() Water is a polar compound. The other hydrogen compounds of Group VIA elements such as H2S, H2Se, and H2Te are compounds that are much less polar than H2O. This explains why water has a much higher boiling point than predicted on the basis of molecular weight alone.
Water is a polar compound. The other hydrogen compounds of Group VIA elements such as H2S, H2Se, and H2Te are compounds that are much less polar than H2O. This explains why water has a much higher boiling point than predicted on the basis of molecular weight alone.
Besides water, certain other hydrogen compounds such as HF and NH3 are also highly polar compounds with especially strong intermolecular attraction. Because elements such as O, F, and N are strongly electronegative, a high degree of polarity is found in these compounds. The result is that the positively charged hydrogen atom of one molecule may be strongly attracted to the negatively charged atom of another molecule. These intermolecular attractions occur throughout the liquid. Because the attractions are especially strong in certain compounds that are made up of hydrogen and electronegative elements, this type of intermolecular attraction is known as hydrogen bonding.
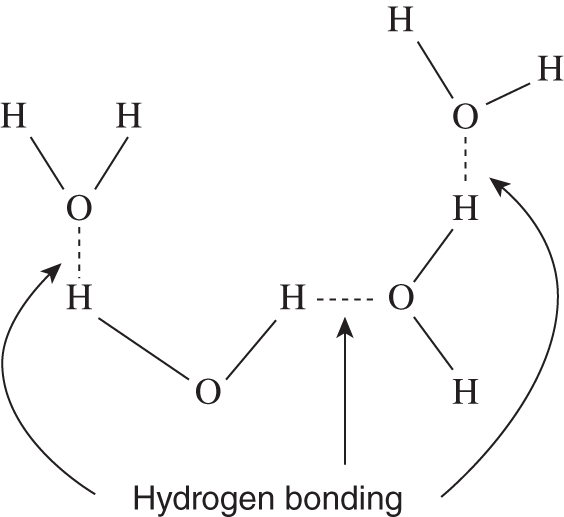
1. Two compounds have similar molecular weights. Hydrogen bonding occurs in one compound but not in the other. Which compound would probably have the higher boiling point, the one with hydrogen bonding or the one without? ___________________
2. Hydrogen bonding, polarity between molecules of a liquid, and van der Waals forces can all be classified as what kind of forces? ________________
Answer: (a) the one with hydrogen bonding; (b) intermolecular attractive forces
![]() Besides affecting the boiling point, intermolecular attractive forces also affect the evaporation of liquids. A liquid with relatively weak intermolecular forces will evaporate more readily than a liquid with relatively strong intermolecular forces. Volatility is a measure of the tendency of liquids to evaporate. A highly volatile liquid evaporates readily.
Besides affecting the boiling point, intermolecular attractive forces also affect the evaporation of liquids. A liquid with relatively weak intermolecular forces will evaporate more readily than a liquid with relatively strong intermolecular forces. Volatility is a measure of the tendency of liquids to evaporate. A highly volatile liquid evaporates readily.
At room temperature, ether (diethyl ether) is highly volatile. Glycerin is very low in volatility. Assume that an equal quantity of each liquid is allowed to evaporate.
1. Which liquid would you expect to evaporate first? ________________
2. Would you expect a liquid with strong intermolecular attractive forces to be highly volatile or low in volatility? ________________
Answer: (a) Ether will evaporate first because of its high volatility. (b) low in volatility
![]() Suppose we have two liquids with similar molecular weights. The liquid with stronger intermolecular attractive forces could be expected to have:
Suppose we have two liquids with similar molecular weights. The liquid with stronger intermolecular attractive forces could be expected to have:
1. (greater, lesser) ___________ viscosity.
2. (greater, lesser) ___________ surface tension.
3. (higher, lower) ___________ boiling point.
4. (higher, lower)___________ volatility.
5. (higher, lower) ___________ vapor pressure.
Answer: (a) greater; (b) greater; (c) higher; (d) lower; (e) lower
MISCIBILITY
![]() The attraction of polar covalent molecules in liquids is one type of intermolecular attraction that affects characteristics such as boiling points, surface tension, volatility, vapor pressure, and viscosity. The polarity of molecules in liquids also affects miscibility, the ability of liquids to mix. Two liquids will mix if both are polar or both are nonpolar. If one liquid is polar and the other is nonpolar, the two liquids will not mix.
The attraction of polar covalent molecules in liquids is one type of intermolecular attraction that affects characteristics such as boiling points, surface tension, volatility, vapor pressure, and viscosity. The polarity of molecules in liquids also affects miscibility, the ability of liquids to mix. Two liquids will mix if both are polar or both are nonpolar. If one liquid is polar and the other is nonpolar, the two liquids will not mix.
Oil and water do not mix. We have learned that water is a polar molecule.
Therefore, would you expect oil to be polar or nonpolar? ______________
Answer: nonpolar
![]() Two polar liquids are miscible; two nonpolar liquids are miscible. However, a polar liquid and a nonpolar liquid are not miscible.
Two polar liquids are miscible; two nonpolar liquids are miscible. However, a polar liquid and a nonpolar liquid are not miscible.
Water is a polar compound. Water and oil are not miscible. Gasoline and oil are miscible. Is gasoline polar or nonpolar? ________________
Answer: nonpolar (Since water and oil are not miscible, oil must be nonpolar. Gasoline is nonpolar because it mixes with oil.)
![]() Immiscible liquids (those not capable of being mixed) generally separate as two layers, with the more dense liquid on the bottom. Gasoline, jet fuel, or oil usually float on top of water because they are less dense than water and are not miscible with water. Based on this information, would you recommend water as a means for putting out gasoline, jet fuel, or oil fires? __________________ Why or why not? ___________________________
Immiscible liquids (those not capable of being mixed) generally separate as two layers, with the more dense liquid on the bottom. Gasoline, jet fuel, or oil usually float on top of water because they are less dense than water and are not miscible with water. Based on this information, would you recommend water as a means for putting out gasoline, jet fuel, or oil fires? __________________ Why or why not? ___________________________
Answer: no; Since these liquids float on top of water, adding water might simply spread the fire. (A more appropriate means for putting out a fire of this type is foam or carbon dioxide, to cut off oxygen to the fire. Foam floats on top of oil or jet fuel.)
![]() We can predict whether liquids are miscible or immiscible by knowing if the liquids are composed of polar or nonpolar molecules. The following table lists some common polar and nonpolar compounds. Read the table carefully and then answer the questions.
We can predict whether liquids are miscible or immiscible by knowing if the liquids are composed of polar or nonpolar molecules. The following table lists some common polar and nonpolar compounds. Read the table carefully and then answer the questions.
Compounds |
Examples |
Polar molecules |
|
H bonded to O, N, F, Br, Cl, and I |
H2O, NH3, HF, HCI |
O bonded to C, F, Cl, Br, I, and other elements |
Alcohols (C2H5OH, CH3OH), glycerine (C3H5(OH)3), ethylene glycol (C2H4(OH)2), HOCI, HOBr, HOI, acetone (CH3COCH3) |
Nonpolar molecules |
|
Linear, planar, or symmetrical compounds with more than two atoms |
CCI4, CF4, CO2, BF3, ether (C2H5OC2H5) |
Containing only C and H |
Gasoline (mixture of C7H16 [heptane], C8H18 [octane], C6H6 [benzene], and C6H5CH3 [toluene]) |
Oils, fats (higher molecular weight containing C, H, and O). |
Waxes, lipids, cooking oils |
1. Is benzene (C6H6) miscible with water (H2O)? ________________
2. A liquid compound made of just C and H is added to a liquid binary compound with strong electronegativity differences. Will the two liquids mix? ________________
3. Is ethylene glycol (C2H4(OH)2) miscible with water (H2O)? ____________
(Note: Ethylene glycol has the structure shown here. The O atoms are bonded to C atoms.)
4. Is the symmetrical compound CCl4 miscible with an oil made up of carbon and hydrogen? ________________
Answer:
1. no (Benzene is a nonpolar compound because it is composed of only carbon and hydrogen. Water is polar since it is composed of H bonded to O.)
2. no (Compounds made of just carbon and hydrogen are nonpolar. Binary compounds with strong electronegativity differences between the two atoms are polar.)
3. yes (Both H2O and C2H4(OH)2 are polar molecules. A mixture of ethylene glycol and water is commonly found in automobile radiators to protect against freezing. These liquids must be miscible in order for the cooling system to be protected.)
4. yes (Both liquids are nonpolar. The CCl4 compound can be used to dissolve oil and grease stains in cloth, although it is now banned for cleaning purposes worldwide because of its effect on the ozone layer of the atmosphere.)
HEAT OF VAPORIZATION
![]() Recall that the heat of fusion is the energy necessary to melt a solid completely to the liquid state (see Chapter 9). While a solid is melting at the melting point through addition of heat of fusion, its temperature does not change. At the boiling point of a liquid, a similar process occurs. In H2O, the boiling point is 100°C at 1 atm pressure. When heated, the temperature of water increases until it reaches 100°C. After water reaches its boiling point, the temperature does not increase until all the liquid has vaporized to steam. The heat required to vaporize water at the boiling point is called the heat of vaporization. The following graph represents the heating of ice cubes until all of the H2O is vaporized.
Recall that the heat of fusion is the energy necessary to melt a solid completely to the liquid state (see Chapter 9). While a solid is melting at the melting point through addition of heat of fusion, its temperature does not change. At the boiling point of a liquid, a similar process occurs. In H2O, the boiling point is 100°C at 1 atm pressure. When heated, the temperature of water increases until it reaches 100°C. After water reaches its boiling point, the temperature does not increase until all the liquid has vaporized to steam. The heat required to vaporize water at the boiling point is called the heat of vaporization. The following graph represents the heating of ice cubes until all of the H2O is vaporized.
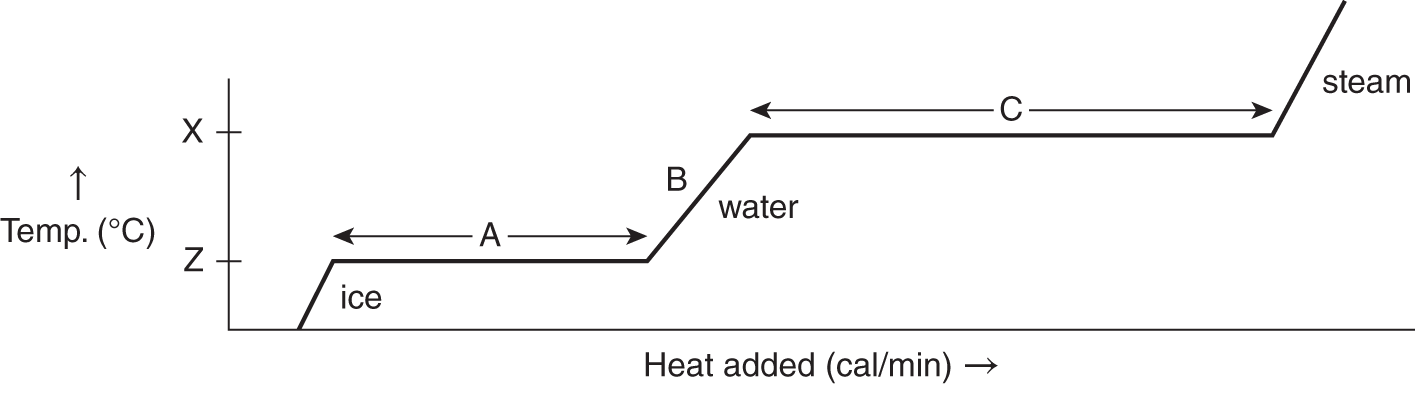
1. What letter represents the heat of vaporization? ______________
2. What letter represents the boiling point? ________________
3. Does the water temperature change while the heat of vaporization is being applied? ________________
Answer: (a) C; (b) X (remember the boiling point is a temperature); (c) no
![]() The graph on the next page represents H2O. At each of the indicated points, tell whether the H2O is solid (ice), liquid (water), gaseous vapor (steam), a mixture of ice and water, or a mixture of water and steam.
The graph on the next page represents H2O. At each of the indicated points, tell whether the H2O is solid (ice), liquid (water), gaseous vapor (steam), a mixture of ice and water, or a mixture of water and steam.
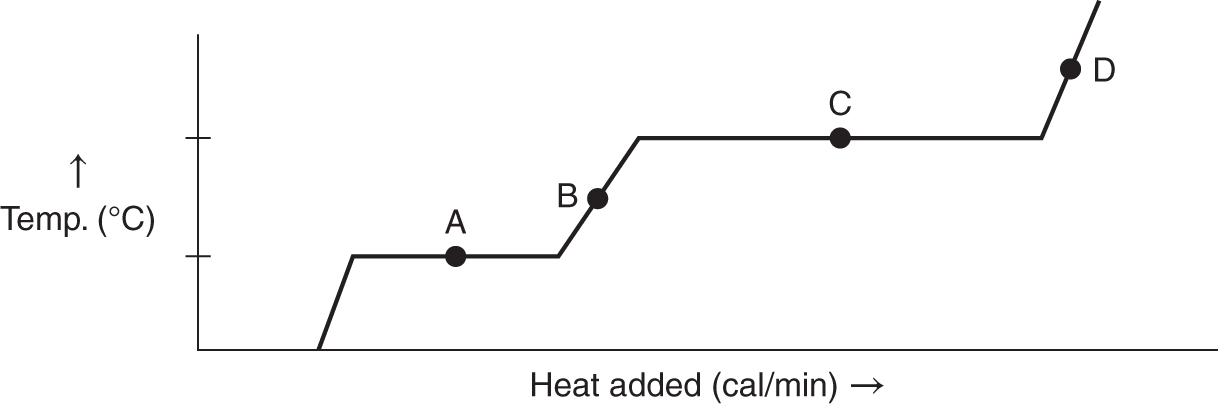
1. Point A ___________________
2. Point B ___________________
3. Point C ___________________
4. Point D ___________________
Answer: (a) ice and water (or solid and liquid); (b) water (or liquid); (c) water and steam (liquid and vapor); (d) steam (vapor)
![]() The heat of vaporization of water is 540 cal/gram. It takes how many calories to convert 1 gram of liquid H2O at 100°C to steam at 100°C? ______________
The heat of vaporization of water is 540 cal/gram. It takes how many calories to convert 1 gram of liquid H2O at 100°C to steam at 100°C? ______________
Answer: 540 cal (This is simply a restatement of the definition of the heat of vaporization.)
![]() The heat of vaporization for water is 540 cal for each gram of H2O. Two grams of water would require twice as many calories.
The heat of vaporization for water is 540 cal for each gram of H2O. Two grams of water would require twice as many calories.
Answer: 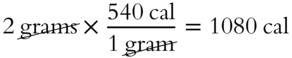
How many calories of heat would be needed to completely vaporize 10 grams of water at the boiling point (100°C)? ________________
Answer: 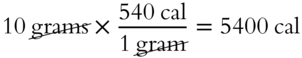
![]() The heat of vaporization is the energy needed to break the intermolecular attractive forces that keep a liquid in liquid form. It takes 540 cal of heat energy to break the intermolecular attractive forces and vaporize 1 gram of liquid H2O at 100°C. It is important to remember that vaporization is a reversible process. When H2O condenses from steam at 100 °C to liquid at 100°C, it releases an amount of energy equal to the heat of vaporization.
The heat of vaporization is the energy needed to break the intermolecular attractive forces that keep a liquid in liquid form. It takes 540 cal of heat energy to break the intermolecular attractive forces and vaporize 1 gram of liquid H2O at 100°C. It is important to remember that vaporization is a reversible process. When H2O condenses from steam at 100 °C to liquid at 100°C, it releases an amount of energy equal to the heat of vaporization.
When 1 gram of steam at 100°C condenses to liquid H2O at 100°C, it releases how many calories of heat? ______________________
Answer: 540 cal
![]() A burn suffered from contact with steam at 100°C is much more severe than a burn suffered from contact with the same quantity of boiling water. Can you explain why, in terms of vaporization? ________________
A burn suffered from contact with steam at 100°C is much more severe than a burn suffered from contact with the same quantity of boiling water. Can you explain why, in terms of vaporization? ________________
Answer: This more severe burn from steam is caused by the release of 540 cal of heat energy per gram when the steam condenses.
![]() Just as a liquid has a characteristic boiling point, each liquid also has a characteristic heat of vaporization. Certain gases that are readily condensed and have high heats of vaporization are useful in such refrigeration systems as air conditioners, refrigerators, or freezers. Refrigeration systems take advantage of the fact that a liquid which is vaporized at its boiling point requires heat. The resulting vapor (gas) releases heat when it condenses again at the boiling point. In a refrigeration system, a gas is compressed to the point where condensation takes place. The heat released by condensation is given off to the surroundings. The coils behind a refrigerator become warm to the touch because heat is being released after condensation. After the liquid releases its heat to the surroundings, it is piped inside the refrigerator and allowed to vaporize and expand very rapidly.
Just as a liquid has a characteristic boiling point, each liquid also has a characteristic heat of vaporization. Certain gases that are readily condensed and have high heats of vaporization are useful in such refrigeration systems as air conditioners, refrigerators, or freezers. Refrigeration systems take advantage of the fact that a liquid which is vaporized at its boiling point requires heat. The resulting vapor (gas) releases heat when it condenses again at the boiling point. In a refrigeration system, a gas is compressed to the point where condensation takes place. The heat released by condensation is given off to the surroundings. The coils behind a refrigerator become warm to the touch because heat is being released after condensation. After the liquid releases its heat to the surroundings, it is piped inside the refrigerator and allowed to vaporize and expand very rapidly.
1. Vaporization is a process that (requires, releases) __________ heat.
2. Condensation is a process that (requires, releases) __________ heat.
Answer: (a) requires; (b) releases
![]() Because the vaporization of the liquid inside the refrigerator piping is a process that requires heat, the heat inside the refrigerator is removed and the food and air inside the refrigerator are cooled. After the gas has removed heat from the inside of the refrigerator, the gas is piped outside and compressed again. The cycle of condensation and vaporization is continued. Refrigeration depends upon the requirement of heat for the process of __________ and the release of heat for the process of __________
Because the vaporization of the liquid inside the refrigerator piping is a process that requires heat, the heat inside the refrigerator is removed and the food and air inside the refrigerator are cooled. After the gas has removed heat from the inside of the refrigerator, the gas is piped outside and compressed again. The cycle of condensation and vaporization is continued. Refrigeration depends upon the requirement of heat for the process of __________ and the release of heat for the process of __________
Answer: vaporization; condensation
![]() The condensation-vaporization cycle is important for refrigeration because heat is taken from one place (inside the refrigerator) through vaporization and released at another place (outside the refrigerator) through condensation. Condensation takes place because the gas is compressed through a mechanical compressor. The compressor forces the gas into less space so that the intermolecular attractive forces are strong enough to overcome the kinetic energy of the gas molecules. The molecules then coalesce and the gas is liquefied.
The condensation-vaporization cycle is important for refrigeration because heat is taken from one place (inside the refrigerator) through vaporization and released at another place (outside the refrigerator) through condensation. Condensation takes place because the gas is compressed through a mechanical compressor. The compressor forces the gas into less space so that the intermolecular attractive forces are strong enough to overcome the kinetic energy of the gas molecules. The molecules then coalesce and the gas is liquefied.
It is possible, however, for the kinetic energy of the molecules to be so great that the intermolecular attractive forces cannot overcome the kinetic energy and the gas will not liquefy no matter how great the pressure. If the coolant gas molecules in a refrigerator possessed so much kinetic energy that the intermolecular attractive forces could not cause the gas to be liquefied no matter how great the pressure, could the refrigerator continue working through the vaporization-condensation cycle? __________ Explain. __________
Answer: no; The gas could not be liquefied under such conditions, and no condensation and therefore no vaporization could take place.
![]() Remember that the temperature is a measure of the average kinetic energy of a gas. The temperature above which the intermolecular attractive forces can no longer overcome the kinetic energy in order to liquefy a gas is known as the critical temperature. At any temperature above the critical temperature, a gas will not liquefy no matter how much pressure is applied.
Remember that the temperature is a measure of the average kinetic energy of a gas. The temperature above which the intermolecular attractive forces can no longer overcome the kinetic energy in order to liquefy a gas is known as the critical temperature. At any temperature above the critical temperature, a gas will not liquefy no matter how much pressure is applied.
If you were selecting a coolant gas for a refrigerator, would you select one with a critical temperature that is well below room temperature or well above? ______________________________________________________
Answer: well above room temperature (The refrigerator will no longer function at any temperature above critical temperature because condensation cannot take place at any temperature above critical temperature. The critical temperature should therefore be well above the normal temperature of the surroundings in which the refrigerator must operate.)
![]() The critical temperature is different for each gas. For example, hydrogen has an extremely low critical temperature of −240°C. In order to liquefy hydrogen gas, the temperature must be (above, at, below) _______ −240°C.
The critical temperature is different for each gas. For example, hydrogen has an extremely low critical temperature of −240°C. In order to liquefy hydrogen gas, the temperature must be (above, at, below) _______ −240°C.
Answer: at or below
SELF-TEST
This self-test is designed to show how well you have mastered this chapter's objectives. Correct answers and review instructions follow the test.
1. Consider two liquids in a chemistry lab, liquid #1 and liquid #2. Liquid #1 flows very quickly when poured out of its beaker while liquid #2 flows very slowly. What conclusion can be made concerning the intermolecular attractive forces for the two liquids?
2. Water bugs walk on water, some razor blades can float on water, and water forms droplets. Are these examples of viscosity, surface tension, or vapor pressure? ________________
3. The process of a liquid changing phases to the gaseous state is known as _____, while ______ describes the process of gaseous molecules changing phases to the liquid state.
4. What principle describes the shift in equilibrium of a chemical system to relieve stress?
5. If we increase the temperature of a liquid, what happens to its vapor pressure? ______________________________________________
6. Below is a graph representing how the vapor pressure of a liquid changes as the temperature increases.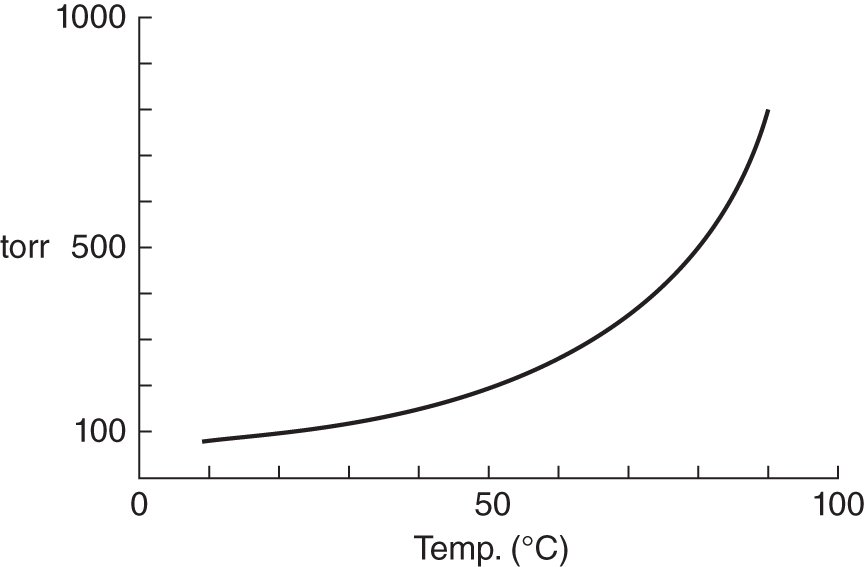
1. What would be the approximate vapor pressure of the liquid at 50°C? ______________________
2. If the pressure on the surface of the liquid is only 500 torr, at what approximate temperature would the liquid boil? ________________
7. Consider the following two liquids, liquid A and liquid B. Liquid A and liquid B are pure liquids. Both liquids are isomers of one another. In other words the molecules that make up both liquids have the same molecular formula but different molecular structures. Liquid A's structure allows for hydrogen bonding while liquid B does not. Which liquid do you predict to have the higher boiling point and why?
8. Water has a normal boiling point of 100°C and ethyl alcohol (46 g/mol) has a normal boiling point of 78°C. Which has the lower intermolecular attractive forces? ____________
9. Containers of three different liquids, A, B, and C, were opened to the air. All three had the same volume to begin with, but the contents of A disappeared very rapidly while the contents of C were the last to disappear. Which liquid has the lowest vapor pressure? _________________
10. Suppose we have two liquids with similar molecular weights. The liquid that has the weaker intermolecular attractive forces could be expected to have:
1. (greater, lesser) ________viscosity.
2. (higher, lower) __________boiling point.
3. (greater, lesser) _________ surface tension.
11. Which of the following compounds would be miscible with water? C3H8, CH3OH, HCl, NH3, C6H6, C12H26.
12. Which would be more serious, a burn from water at 100°C or a burn from steam at 100°C? _____________ Why? ________________
13. Below is a graph representing a liquid being heated at a constant rate. What is happening at B and C? ________________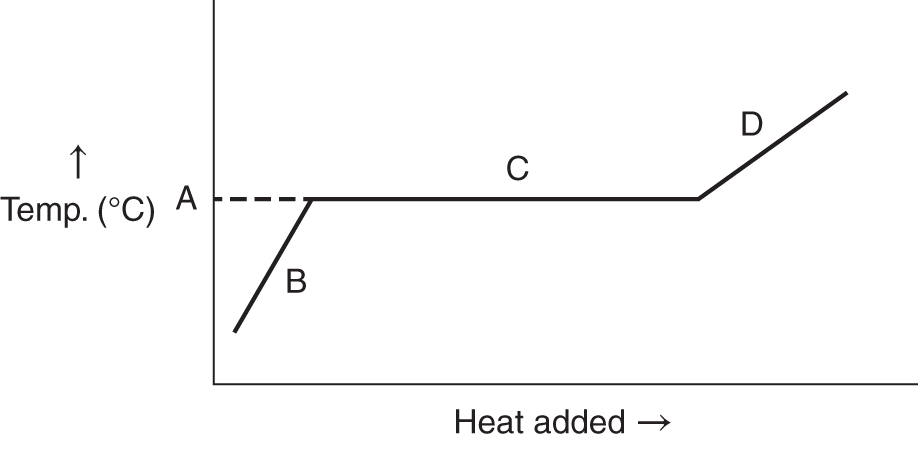
14. A liquid was vaporized in a closed container at a temperature of 200°C. The container was such that one end could be pushed in to increase the pressure on the vapor. While maintaining the temperature of the vapor at 200°C, the pressure on the vapor was increased to 1000 atm but none of the vapor condensed (liquefied). The 200°C temperature is most likely above the _________________ temperature of the gas.
15. Neon gas has an extremely low critical temperature of -229oC. In order to liquefy neon gas, the temperature must be (above, at, below) -229oC.
ANSWERS
Compare your answers to the self-test with those given below. If you answer all questions correctly, you are ready to proceed to the next chapter. If you miss any, review the frames indicated in parentheses following the answers. If you miss several questions, you should probably reread the chapter carefully.
1. The more intermolecular forces the molecules of liquid possess, the slower the flow rate. Conversely, the less intermolecular forces molecules have the faster the flow rate. Liquid #1 must have few intermolecular attractive forces than those found in liquid #2. This would explain why liquid #1 flows faster than liquid #2. [frames 3-5]
2. surface tension (frames 6, 7)
3. evaporation, condensation [frames 9, 12]
4. Le Chatelier's Principle [frame 14]
5. Vapor pressure will increase. (frames 16, 20)
6. (a) approximately 200 torr; (b) approximately 80°C (frame 20)
7. Liquid A is expected to have the higher boiling point since it has hydrogen bonding interactions. [frames 18-24]
8. alcohol (It has the lower boiling point.) (frame 23)
9. C. The containers held three liquids with different boiling points, vapor pressures, and degree of volatility. Container A held the liquid that was most volatile and had the highest vapor pressure and lowest boiling point. Container C held the liquid that was least volatile and had the lowest vapor pressure and highest boiling point. Container B held a liquid with properties somewhere between those of A and C. (frame 25)
10. (a) lesser, (b) lower, (c) lesser [frames 3-22]
11. CH3OH, HCl, NH3 [frames 27-30]
12. steam; Steam has 540 cal more heat per gram at 100 °C (heat of vaporization) than water does at 100°C. (frames 31, 36)
13. At B the temperature of the liquid is increasing as heat is being added. At C the liquid is boiling while the temperature remains constant. The flat portion represents heat of vaporization. (frames 31, 32)
14. critical (frames 40, 41)
15. at or below [frame 41]
THE LIQUID STATE
Liquids are one of the three common states of matter. Molecules in a liquid are held loosely together, and not nearly as closely as those found in solids. In fact, molecules in the liquid state are able to have reasonable contact with one another but the distances between each molecule are farther apart than those found in solids. However, the freedom of their movement allows them to take the shape of the container they fill.
Although there is more distance between the molecules in the liquid state that distance does not even come close to that found between gas particles and molecules. In the gaseous state, the third state of matter, particles are often several atoms or molecules away from one another.
Liquids are very important for life processes. For instance, our cellular fluid, gastric juices, and saliva all work within a liquid medium. The liquid that makes up these biological solutions is water, and water is essential for all life. Without water, life as we know it would not exist. Water is a very special liquid.
One of the amazing properties of water is that it is a liquid on Earth. This is incredible since water has a molecular mass of 18.02 g/mol. Why is this so important? Smaller molecules have fewer dispersion forces than larger ones. Dispersion forces are one of several intermolecular forces that help attract molecules to one another. The larger the molecule (i.e., the more dispersion forces it has) the more likely it will be a liquid or solid at room temperature.
Water is a very small molecule yet it can exist as a liquid on our planet where the atmospheric pressure at sea level is one atmosphere (1 atm). Even more remarkable is the wide range of temperatures on our planet. Earth can be rather hot, especially around the equator. Having liquid water is even more remarkable when considering how hot it can get here! Let's look into this further to help understand why having water on Earth is so amazing.
Many compounds that have a greater molar mass (i.e., more dispersion forces) are actually gases at 1 atm of pressure at room temperature. For instance, carbon monoxide and carbon dioxide are both gases at 1 atm. Carbon monoxide has a molar mass of 28.01 g/mol whereas the molar mass of carbon dioxide is 44.01 g/mol.
Other compounds that exist as gases at 1 atm and room temperature include chlorine (Cl2, molar mass 70.90 g/mol), propane (C3H8, molar mass 44.10 g/mol), ozone (O3, molar mass 48.00 g/mol), and phosgene (COCl2, molar mass 98.91 g/mol). As you can see, especially in phosgene's case, water's molar mass is significantly less, yet it is a liquid and not a gas at room temperature and 1 atm.
What makes water a liquid under our normal conditions? The answer is that it has an important additional intermolecular force known as hydrogen bonding. The oxygen atom is electronegative and it is single bonded to two hydrogen atoms. The oxygen atom of one water molecule is attracted to the hydrogen atom of another water molecule.
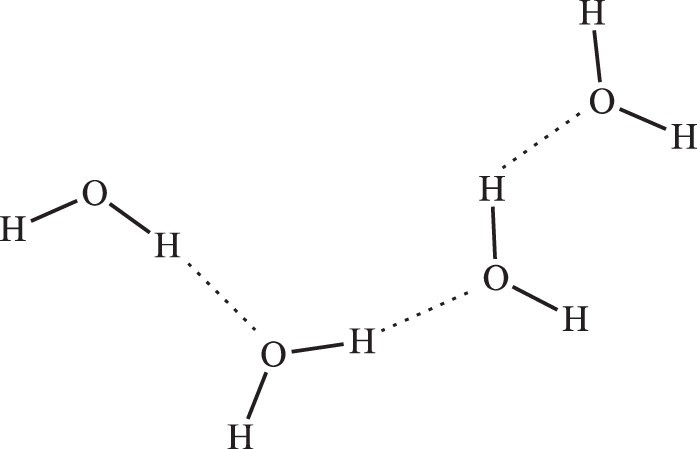
The number of hydrogen bonds to water varies with temperature. It can be as high as four hydrogen bonds per water molecule or a low as one. At lower temperatures water experiences more hydrogen bonding per molecule, while at higher temperatures there is a decrease in the number of hydrogen bonding interactions.
Hydrogen bonding is a rather weak intermolecular force by itself, but when the number of hydrogen bonding interactions adds up over enough water molecules the effects are significant. In fact, the sum of hydrogen bonding interactions is the main reason why we can have liquid water on our planet! Instead of boiling at temperatures lower than 0°C, water has a boiling point of 100°C, all thanks to hydrogen bonding interactions!
The unique nature of water does not end there. In a 2019 Nature publication researchers discovered superionic water, aka ice XVIII. Ice XVIII is a unique phase of water that exists at extremely high temperatures and pressures. In this phase water molecules are broken down into oxide ions (O2−) that crystallize into an oxygen lattice where the hydrogen ions move around freely within it. As interesting as this sounds, these freely moving hydrogen ions make superionic water as conductive as metals!