Chemistry: A Self-Teaching Guide - Post R., Snyder C., Houk C.C. 2020
Solutions and Their Properties
You have just been introduced to the three states of matter — solids, liquids, and gases — and their “observable” properties. Most substances that you encounter in your daily existence are mixtures of these states of matter. The mixtures may be solids in liquids, liquids in liquids, gases in liquids, gases in gases, or any combination of the three states. The most common mixtures are solids in liquids.
This chapter deals with the most common of all liquids, water, and the properties of mixtures of water with other states of matter, particularly solids. This chapter also is concerned with the ways of expressing the quantitative relationships between mixtures of states of matter.
OBJECTIVES
After you have completed this chapter, you will be able to
· recognize and apply or illustrate: solute, solvent, dilute, concentrated, molarity, molality, mole fraction, weight percent, solubility, hydrate, hydration, salvation, saturated, unsaturated, supersaturated, electrolyte, nonelectrolyte, hygroscopic, Raoult's Law, acid anhydride, and basic anhydride;
· given all but one of the following data, calculate the remaining unknown data: molecular weight of the solute, number of grams of the solute, number of grams of solvent (or density and volume), and molality of the solution;
· given all but one of the following data, calculate the remaining unknown data: the molality of a solution (or the data needed to find it), the molal freezing point depression constant, and the lowering of the freezing point of the solution (or the data needed to find it);
· given all but one of the following data, but with the molal boiling point elevation constant, calculate: the rise in the boiling point of the solution, the molality of a solution (or the data needed to find it), the molal freezing point depression constant, and the lowering of the freezing point of the solution (or the data needed to find it);
· given all but one of the following data, calculate the remaining unknown data: number of moles of solute, molarity of the solution, and volume of the solution;
· be able to write balanced chemical equations for the reaction of water with oxides of the alkali metals and oxides of the alkaline earth metals.
SALTS AND OXIDES
![]() A solution is a homogeneous mixture of two or more substances. The solution looks the same throughout, as though it consists of only one substance. No different layers are visible in a true solution. Lemonade, tea, and soda pop are some common solutions made of water and other chemical substances.
A solution is a homogeneous mixture of two or more substances. The solution looks the same throughout, as though it consists of only one substance. No different layers are visible in a true solution. Lemonade, tea, and soda pop are some common solutions made of water and other chemical substances.
A solution is composed of one or more solutes and one solvent. A solute is a substance that is dissolved and is usually the substance of lesser quantity in a solution. The solvent is the substance doing the dissolving and of greater quantity in a solution.
Suppose a teaspoon of table salt is dissolved in a glass of water.
1. Which is the solute, water or salt? __________
2. Which is the solvent? __________
3. From your experience, is the resulting mixture a solution? __________
Answer: (a) salt; (b) water; (c) yes (it appears the same throughout and has no visible layers)
![]() In a sugar and water solution, sugar is the __________ and water is the _____________.
In a sugar and water solution, sugar is the __________ and water is the _____________.
Answer: solute; solvent
![]() Various types of solutions can include gases dissolved in liquids, liquids in liquids, or solids in liquids. A salt-water solution is an example of a solution involving a solid dissolved in a liquid.
Various types of solutions can include gases dissolved in liquids, liquids in liquids, or solids in liquids. A salt-water solution is an example of a solution involving a solid dissolved in a liquid.
In a fish aquarium, an electric pump is often used to bubble air in the aquarium water. Some of the oxygen remains dissolved in the water. This solution is an example where a __________ is the solute and a __________ is the solvent.
Answer: gas; liquid (The solution consists of a gas dissolved in a liquid.)
![]() The most common solvent is water. The most common solutes dissolved in water are the soluble solids encountered in Chapter 6.
The most common solvent is water. The most common solutes dissolved in water are the soluble solids encountered in Chapter 6.
A soluble ionic solid in aqueous solution is called an electrolyte because such a solution conducts electricity. In aqueous (water) solution, the ions of an ionic compound dissociate and are free to move about to conduct electricity. A nonelectrolyte may also dissolve, but the solute remains in molecular form and will not conduct electricity.
1. An aqueous solution of NaCl conducts electricity. Is the solute NaCl an electrolyte or nonelectrolyte? __________
2. A solute made up of dissociated positive and negative ions is a(n) (electrolyte, nonelectrolyte) __________
3. Does a solute that remains in molecular form conduct electricity? __________
Answer: (a) electrolyte; (b) electrolyte; (c) no
![]() Instead of a solute just dissolving in water, it may react with water to form a different compound. Oxides of alkali metals (Na2O, K2O, Li2O) and of alkaline earth metals (MgO, CaO, BaO) react with water to form metal hydroxide solutions. For example:
Instead of a solute just dissolving in water, it may react with water to form a different compound. Oxides of alkali metals (Na2O, K2O, Li2O) and of alkaline earth metals (MgO, CaO, BaO) react with water to form metal hydroxide solutions. For example:
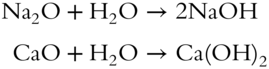
Sodium hydroxide, NaOH, ionizes to yield Na+ and OH−. Calcium hydroxide, Ca(OH)2, ionizes to yield Ca2+ and 2OH−.
A compound that ionizes to form OH− in aqueous solution is called a base. After the metal oxide has reacted with the water, there is actually a new solute in the solution, the metal hydroxide. In the equations above, what compounds are the solutes once the reaction has occurred? __________
Answer: NaOH (or Na+ and OH—) and Ca(OH)2 (or Ca2+ and 2OH—)
![]() The general equation for the reaction of an alkali metal oxide with water is as follows. (Substitute any alkali metal for “M.”)
The general equation for the reaction of an alkali metal oxide with water is as follows. (Substitute any alkali metal for “M.”)
![]()
(MOH is a base since it dissociates to form OH− ions.)
When K2O is mixed with an excess of water, the resulting aqueous solution contains what base as the solute? __________
Answer: KOH (K+, OH−)
![]() The general equation for the reaction of any alkaline earth metal oxide with water is as follows. (Substitute any alkaline earth metal for “M.”)
The general equation for the reaction of any alkaline earth metal oxide with water is as follows. (Substitute any alkaline earth metal for “M.”)
![]()
When MgO is mixed in an excess of water, the resulting aqueous solution contains __________ as the solute.
Answer: Mg(OH)2 (Mg2+, 2OH−). Note that the oxidation number of the metal ion does not change in going from metal oxide to metal hydroxide.
![]() Many nonmetal oxides react with water to form acids that are ionized in aqueous solutions, yielding H+ ions and a negative ion. A new solute is the result.
Many nonmetal oxides react with water to form acids that are ionized in aqueous solutions, yielding H+ ions and a negative ion. A new solute is the result.
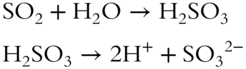
In the above reaction the resulting solution contains __________ as the solute.
Answer: H2SO3 (2H+, SO32−)
![]() The oxides of a number of nonmetals such as carbon, sulfur, nitrogen, and phosphorus react with water to form acids. When the nonmetal oxides are mixed in water, the resulting solute is an acid because of the reaction of the oxide with the water.
The oxides of a number of nonmetals such as carbon, sulfur, nitrogen, and phosphorus react with water to form acids. When the nonmetal oxides are mixed in water, the resulting solute is an acid because of the reaction of the oxide with the water.
![]()
What is the new solute in this reaction? __________
Answer: HNO3 (H+ and NO3− ions)
![]() What is the resulting solute in the following mixture of carbon dioxide and water?
What is the resulting solute in the following mixture of carbon dioxide and water?
![]()
Answer: H2CO3 (2H+, CO32−)
![]() Nonmetal oxides (such as N2O5, SO2, SO3, P2O3, P2O5, CO2, and so on) react with water to form acids. Note that the nonmetal does not change oxidation number in going from the oxide to the acid. The formulas of some of the acids are obtained simply by “adding” the atoms together. Here are some obvious examples.
Nonmetal oxides (such as N2O5, SO2, SO3, P2O3, P2O5, CO2, and so on) react with water to form acids. Note that the nonmetal does not change oxidation number in going from the oxide to the acid. The formulas of some of the acids are obtained simply by “adding” the atoms together. Here are some obvious examples.
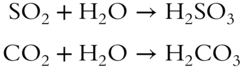
Others are not as easy to see.
![]()
We must rely upon the formulas and names of the common acids you learned in Chapter 5.
What acid is formed when SO3 reacts with H2O? __________
Answer: sulfuric acid, H2SO4 (H2O + SO3 → H2SO4)
![]() An unexpected product is sometimes formed when an automobile is equipped with a catalytic converter. The catalytic converter allows sulfur dioxide and sulfur trioxide and water vapor to mix together and react. The reaction of water and sulfur trioxide produces a pollutant, which is then sprayed out of the automobile exhaust system. Identify the pollutant. __________
An unexpected product is sometimes formed when an automobile is equipped with a catalytic converter. The catalytic converter allows sulfur dioxide and sulfur trioxide and water vapor to mix together and react. The reaction of water and sulfur trioxide produces a pollutant, which is then sprayed out of the automobile exhaust system. Identify the pollutant. __________
Answer: H2SO4 (sulfuric acid)
![]() Nonmetal oxides are known as acid anhydrides (acids without water) because they form acids when added to water. Metal oxides are known as basic anhydrides (bases without water) because they form bases when added to water.
Nonmetal oxides are known as acid anhydrides (acids without water) because they form acids when added to water. Metal oxides are known as basic anhydrides (bases without water) because they form bases when added to water.
Identify the following as either acid anhydrides or basic anhydrides.
1. MgO __________
2. P2O5 __________
3. N2O3 __________
4. BaO __________
Answer: (a) basic anhydride; (b) acid anhydride; (c) acid anhydride; (d) basic anhydride
![]() Acid anhydride + H2O → ________. Basic anhydride + H2O → _________
Acid anhydride + H2O → ________. Basic anhydride + H2O → _________
Answer: acid; base
HYDRATES AND WATER OF HYDRATION
![]() Certain compounds actually contain water within the structure of their crystal lattices. Those compounds are called hydrates. The water they contain is known as the water of hydration. Probably the best known hydrate is copper sulfate pentahydrate, which has a characteristic deep blue color and is symbolized as CuSO4 · 5H2O. The dot between CuSO4 and 5H2O means that water is actually a part of the hydrate molecule, and the formula weight of the hydrate must include the water.
Certain compounds actually contain water within the structure of their crystal lattices. Those compounds are called hydrates. The water they contain is known as the water of hydration. Probably the best known hydrate is copper sulfate pentahydrate, which has a characteristic deep blue color and is symbolized as CuSO4 · 5H2O. The dot between CuSO4 and 5H2O means that water is actually a part of the hydrate molecule, and the formula weight of the hydrate must include the water.
A hydrate is a compound containing what as a part of its crystal lattice? __________
Answer: water (H2O)
![]() Determine the formula weight in grams (to the nearest tenth) of copper sulfate pentahydrate. (You might want to make a table like the ones we used in Chapter 4 to determine formula weights. If so, use a separate sheet of paper to set up the table and calculate.)
Determine the formula weight in grams (to the nearest tenth) of copper sulfate pentahydrate. (You might want to make a table like the ones we used in Chapter 4 to determine formula weights. If so, use a separate sheet of paper to set up the table and calculate.)
Formula weight of CuSO4 · 5H2O = ________
Answer:
Element |
Number of atoms |
Gram atomic weight |
Atoms × weight |
Cu |
1 Cu |
63.5 |
1 × 63.5 = 63.5 |
S |
1 S |
32.1 |
1 × 32.1 = 32.1 |
O |
9 O |
16.0 |
9 × 16.0 = 144.0 |
H |
10 H |
1.0 |
10 × 1.0 = 10.0 |
Formula weight is 249.6 grams |
![]() It is possible to determine the percentage that the water contributes to the total weight of a hydrate. Just divide the weight of the water molecules by the entire formula weight of the hydrate (from the previous frame) and multiply the result by 100. For copper sulfate pentahydrate, divide the weight of 5 mol of water by the formula weight of the entire hydrate and multiply by 100.
It is possible to determine the percentage that the water contributes to the total weight of a hydrate. Just divide the weight of the water molecules by the entire formula weight of the hydrate (from the previous frame) and multiply the result by 100. For copper sulfate pentahydrate, divide the weight of 5 mol of water by the formula weight of the entire hydrate and multiply by 100.
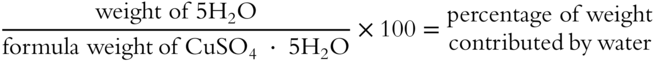
The percentage of weight contributed by water in CuSO4 · 5H2O is __________ (Round this and all other answers in this chapter to the nearest tenth unless otherwise indicated.)
Answer: The weight of 5H2O is:
Element |
Number of atoms |
Gram atomic weight |
Atoms × weight |
H |
10 H |
1.0 |
10 × 1.0 = 10.0 |
O |
5 O |
16.0 |
5 × 16.0 = 80.0 |
Formula weight is 90.0 grams |
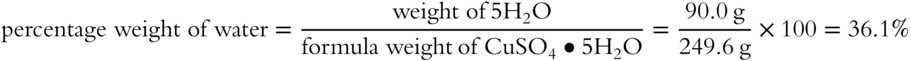
![]() Another hydrate is magnesium sulfate heptahydrate, more commonly known as Epsom salts. Determine the formula weight in grams of this hydrate: MgSO4 · 7H2O. __________
Another hydrate is magnesium sulfate heptahydrate, more commonly known as Epsom salts. Determine the formula weight in grams of this hydrate: MgSO4 · 7H2O. __________
Answer: 246.4 grams
![]() The percentage by weight of water in Epsom salts (MgSO4 · 7H2O) is _______________%. (The weight of 7 mol of H2O is 7 × 18.0 = 126.0 grams).
The percentage by weight of water in Epsom salts (MgSO4 · 7H2O) is _______________%. (The weight of 7 mol of H2O is 7 × 18.0 = 126.0 grams).
Answer: 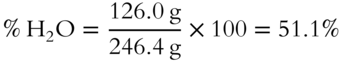
![]() When heated sufficiently, a hydrate will release all of its water molecules. Copper sulfate pentahydrate (CuSO4 · 5H2O) is a deep blue color. Upon heating (symbolized by Δ), it loses its water molecules and becomes white.
When heated sufficiently, a hydrate will release all of its water molecules. Copper sulfate pentahydrate (CuSO4 · 5H2O) is a deep blue color. Upon heating (symbolized by Δ), it loses its water molecules and becomes white.
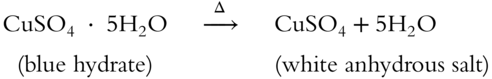
Upon heating, 1 mol of CuSO4 · 5H2O yields 1 mol of CuSO4 · How many mole(s) of H2O result? __________
Answer: 5
![]() The white CuSO4 is the anhydrous (waterless) salt of the CuSO4 · 5H2O hydrate. In the presence of water, the white anhydrous CuSO4 absorbs water into its crystal lattice and returns to the blue hydrate (CuSO4 · 5H2O). Every mole of CuSO4 absorbs 5 mol of H2O to form 1 mol of CuSO4 · 5H2O hydrate. One mole of water weighs 18.0 grams. One mole of CuSO4 absorbs how many grams of H2O to form 1 mol of CuSO4 · 5H2O hydrate? __________
The white CuSO4 is the anhydrous (waterless) salt of the CuSO4 · 5H2O hydrate. In the presence of water, the white anhydrous CuSO4 absorbs water into its crystal lattice and returns to the blue hydrate (CuSO4 · 5H2O). Every mole of CuSO4 absorbs 5 mol of H2O to form 1 mol of CuSO4 · 5H2O hydrate. One mole of water weighs 18.0 grams. One mole of CuSO4 absorbs how many grams of H2O to form 1 mol of CuSO4 · 5H2O hydrate? __________
Answer: 90.0 (5 × 18.0)
![]() Suppose you had 3 mol of CuSO4 · 5H2O and heated it. Three moles of CuSO4 · 5H2O hydrate produces how many moles of H2O when heated? __________ How many grams of H2O? __________
Suppose you had 3 mol of CuSO4 · 5H2O and heated it. Three moles of CuSO4 · 5H2O hydrate produces how many moles of H2O when heated? __________ How many grams of H2O? __________
Answer: 15; 270.0 (15 × 18.0)
![]() If 1 kilogram of CuSO4 · 5H2O hydrate is heated, how many grams of water are produced? (Hint: Convert the kilogram of CuSO4 · 5H2O to moles of CuSO4 · 5H2O.)
If 1 kilogram of CuSO4 · 5H2O hydrate is heated, how many grams of water are produced? (Hint: Convert the kilogram of CuSO4 · 5H2O to moles of CuSO4 · 5H2O.)
Answer:
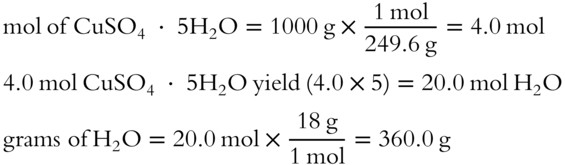
![]() Suppose we know that Epsom salt is a hydrate, but we don't know the number of molecules of water that are part of the hydrate formula. In the following equation, a known weight of Epsom salt hydrate is heated. The resulting anhydrous MgSO4 is weighed.
Suppose we know that Epsom salt is a hydrate, but we don't know the number of molecules of water that are part of the hydrate formula. In the following equation, a known weight of Epsom salt hydrate is heated. The resulting anhydrous MgSO4 is weighed.
![]()
Upon heating, the hydrate loses its water. To determine the weight of water lost, subtract the weight of ___________ from the weight of __________
Answer: MgSO4; MgSO4 · ? H2O
![]() In our equation from frame 24, if the weight of H2O is known and the weight of anhydrous MgSO4 is known, both weights can be converted to moles. The resulting mole fractions can be converted to simple whole numbers to determine the proper formula. Suppose that 3.5 mol of H2O and 0.5 mol of MgSO4 were produced when MgSO4 · ?H2O was heated. The appropriate formula for the hydrate would be MgSO4 · ___H2O. (Divide each mole result by the smallest mole result to convert both to simple whole numbers.)
In our equation from frame 24, if the weight of H2O is known and the weight of anhydrous MgSO4 is known, both weights can be converted to moles. The resulting mole fractions can be converted to simple whole numbers to determine the proper formula. Suppose that 3.5 mol of H2O and 0.5 mol of MgSO4 were produced when MgSO4 · ?H2O was heated. The appropriate formula for the hydrate would be MgSO4 · ___H2O. (Divide each mole result by the smallest mole result to convert both to simple whole numbers.)
Answer: 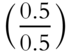 = 1 and
= 1 and 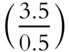 = 7
= 7
The formula is MgSO4 · 7H2O.
![]() The hydrate BaCl2 · ?H2O is heated to give:
The hydrate BaCl2 · ?H2O is heated to give:
![]()
A sample of the hydrate weighed 488.4 grams before heating. After heating, the anhydrous BaCl2 weighed 416.4 grams. Determine:
1. the weight of the water formed. ___________________
2. the moles of water formed. __________
3. the moles of BaCl2 formed. __________
4. the true hydrate formula. __________
Answer:
1. weight of H2O = 488.4 g − 416.4 g = 72.0 g H2O
2. mol H2O = 72.0 g × 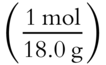 = 4 mol H2O
= 4 mol H2O
3. mol BaCl2 = 416.4 g × 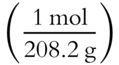 = 2 mol BaCl2
= 2 mol BaCl2
4. 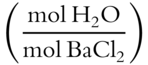 =
= ![]() = 2
= 2
The formula is BaCl2 · 2H2O.
![]() What does the dot (·) between BaCl2 and 2H2O in the BaCl2 · 2H2O hydrate indicate? ____________
What does the dot (·) between BaCl2 and 2H2O in the BaCl2 · 2H2O hydrate indicate? ____________
Answer: that water is an integral part of the crystal lattice of the hydrate (See frame 15 for review.)
![]() Some chemical substances have the ability to absorb moisture from the atmosphere. Such substances are said to be hygroscopic. Flour and table salt are hygroscopic. White anhydrous CuSO4 absorbs water to form the blue hydrate CuSO4 · 5H2O. Because of this very distinct color change, solid anhydrous CuSO4 is used to detect the presence of water in other liquids. If the solid turns from white to blue when added to a liquid, water is present.
Some chemical substances have the ability to absorb moisture from the atmosphere. Such substances are said to be hygroscopic. Flour and table salt are hygroscopic. White anhydrous CuSO4 absorbs water to form the blue hydrate CuSO4 · 5H2O. Because of this very distinct color change, solid anhydrous CuSO4 is used to detect the presence of water in other liquids. If the solid turns from white to blue when added to a liquid, water is present.
Some anhydrous salts absorb enough water to dissolve themselves in their own water of hydration. They are called deliquescent. Anhydrous CaCl2 is such a substance and is often used on dusty dirt roads or race tracks to control the dust by keeping it moist.
Substances that absorb moisture from the atmosphere are __________.
Answer: hygroscopic (deliquescent if they absorb enough to dissolve in it)
KINDS OF SOLUTIONS
![]() There is an upper limit to the amount of substance that a quantity of solvent can dissolve depending upon the type of solvent, the substance to be dissolved, and the temperature. A solution containing the maximum amount of solute at a given temperature is said to be saturated. Additional solute material will not dissolve in a saturated solution. An unsaturated solution contains less than the maximum amount of solute at a given temperature. Additional solute material will dissolve in an unsaturated solution.
There is an upper limit to the amount of substance that a quantity of solvent can dissolve depending upon the type of solvent, the substance to be dissolved, and the temperature. A solution containing the maximum amount of solute at a given temperature is said to be saturated. Additional solute material will not dissolve in a saturated solution. An unsaturated solution contains less than the maximum amount of solute at a given temperature. Additional solute material will dissolve in an unsaturated solution.
Half a gram of NaCl, table salt, is added to an aqueous solution of NaCl. The added salt dissolves. What kind of solution is it, saturated or unsaturated? ___________________
Answer: unsaturated
![]() A solution can also be supersaturated, that is, made to hold more dissolved solute than normally expected at a given temperature. This can occur when a saturated solution is slowly cooled. The cooler solution then holds more dissolved solute than normal. If an additional crystal of solute is added to a supersaturated solution, the excess dissolved solute will quickly crystallize and come out of solution. After the excess solute comes out of solution, the remaining solution is saturated at the new temperature.
A solution can also be supersaturated, that is, made to hold more dissolved solute than normally expected at a given temperature. This can occur when a saturated solution is slowly cooled. The cooler solution then holds more dissolved solute than normal. If an additional crystal of solute is added to a supersaturated solution, the excess dissolved solute will quickly crystallize and come out of solution. After the excess solute comes out of solution, the remaining solution is saturated at the new temperature.
A crystal of NaCl is added to an aqueous solution of NaCl. The crystal will not dissolve but has no effect on the solution. What kind of solution is it— unsaturated, saturated, or supersaturated? __________
Answer: saturated
![]() A crystal of sodium thiosulfate is added to an aqueous sodium thiosulfate solution. Immediately, some of the solute crystallizes out of solution.
A crystal of sodium thiosulfate is added to an aqueous sodium thiosulfate solution. Immediately, some of the solute crystallizes out of solution.
1. What kind of solution was it before the crystal was added? __________
2. What kind of solution is it after the solute crystallizes? __________
Answer: (a) supersaturated; (b) saturated
![]() A solution with a relatively large amount of solute in a given amount of solvent is considered to be a concentrated solution. Concentrated is not a very precise term. It simply means that a relatively large proportion of the solution is made up of solute.
A solution with a relatively large amount of solute in a given amount of solvent is considered to be a concentrated solution. Concentrated is not a very precise term. It simply means that a relatively large proportion of the solution is made up of solute.
A solution with a relatively small amount of solute in a given amount of solvent is considered to be a dilute solution. Dilute means that a relatively small proportion of the solution is made up of solute.
Would a saturated solution of NaCl be considered concentrated or dilute? _______________
Answer: concentrated (because it contains the maximum amount of NaCl)
![]() A small amount of concentrated aqueous sodium chloride solution is added to a very large amount of solvent (water). The resulting aqueous solution is probably (concentrated, dilute) __________
A small amount of concentrated aqueous sodium chloride solution is added to a very large amount of solvent (water). The resulting aqueous solution is probably (concentrated, dilute) __________
Answer: dilute
From this point on in this chapter, we do a great deal of manipulation of mathematical formulas and continue to use dimensional analysis in our problem solving. We suggest you obtain assistance if you have difficulty.
PROPORTIONS IN SOLUTIONS
![]() It is useful to know precisely how dilute or concentrated a solution is. There are several methods of showing the proportion of solute to the solvent. One method of showing precise proportions is to give percent by weight. For example, a 10% solution of NaCl in water indicates that 10% of the solution weight is attributed to NaCl and 90% of the solution weight is attributed to the solvent.
It is useful to know precisely how dilute or concentrated a solution is. There are several methods of showing the proportion of solute to the solvent. One method of showing precise proportions is to give percent by weight. For example, a 10% solution of NaCl in water indicates that 10% of the solution weight is attributed to NaCl and 90% of the solution weight is attributed to the solvent.
In 100 grams of solution that is 10% NaCl by weight, there are how many grams of NaCl? _________ How many grams of water? __________
Answer:

![]() Our NaCl solution from frame 34 gives us the following:
Our NaCl solution from frame 34 gives us the following:
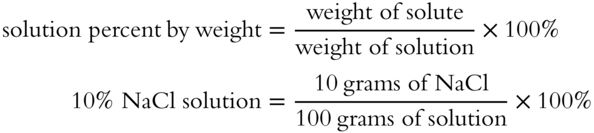
Determine the percent by weight of a solution of NaCl if there are 30 grams of NaCl dissolved in water to yield a total solution weight of 200 grams. Remember, in this case, the “part” is the solute weight and the “whole” is the total weight of the solution: percent = (part/whole) × 100
Answer: 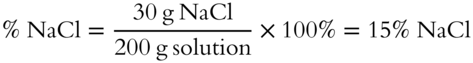
![]() Twenty-five grams of NaOH (solute) are added to 175 grams of water (solvent). What is the % NaOH in the solution? __________ (Note: The total solution weight includes the weight of both solvent and solute.)
Twenty-five grams of NaOH (solute) are added to 175 grams of water (solvent). What is the % NaOH in the solution? __________ (Note: The total solution weight includes the weight of both solvent and solute.)
Answer: total solution weight = 25 grams of solute + 175 grams of solvent = 200 grams
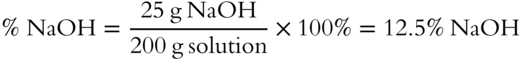
![]() In 100 grams of an aqueous solution that is 15% NaCl by weight, there are how many grams of NaCl? __________ How many grams of water? __________
In 100 grams of an aqueous solution that is 15% NaCl by weight, there are how many grams of NaCl? __________ How many grams of water? __________
Answer:
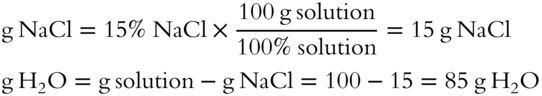
![]() Concentrated HCl is actually an aqueous solution. Concentrated HCl is 37.9% by weight HCl. How many grams of concentrated 37.9% HCl solution does it take to yield 10 grams of HCl (solute)? (The grams of solution is the unknown.)
Concentrated HCl is actually an aqueous solution. Concentrated HCl is 37.9% by weight HCl. How many grams of concentrated 37.9% HCl solution does it take to yield 10 grams of HCl (solute)? (The grams of solution is the unknown.)
Answer: 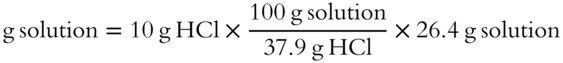
![]() Let's try another one. What weight of dilute nitric acid solution contains 20 grams of HNO3? The solution is 19% HNO3 by weight. The total weight of the solution is __________ grams.
Let's try another one. What weight of dilute nitric acid solution contains 20 grams of HNO3? The solution is 19% HNO3 by weight. The total weight of the solution is __________ grams.
Answer: 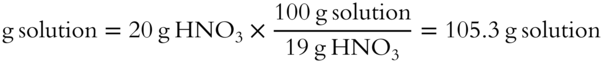
![]() It is also possible to determine the volume of the solution if both the weight and the density of the solution are known.
It is also possible to determine the volume of the solution if both the weight and the density of the solution are known.
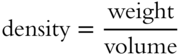
Weight is usually expressed in grams and volume in milliliters (mL). If a solution has a density of 1.5 g/mL, how much volume would 3 grams of the solution occupy? __________
Answer:
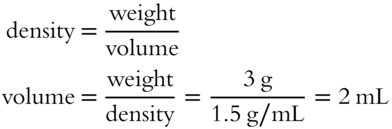
![]() In frame 39, it was determined that a weight of solution of 105.3 grams contained 20 grams of HNO3 in a 19% aqueous HNO3 solution. The total solution weighs 105.3 grams. The density of 19% HNO3 solution is 1.11 g/mL. The volume occupied by 105.3 g at 1.11 g/mL is __________ mL.
In frame 39, it was determined that a weight of solution of 105.3 grams contained 20 grams of HNO3 in a 19% aqueous HNO3 solution. The total solution weighs 105.3 grams. The density of 19% HNO3 solution is 1.11 g/mL. The volume occupied by 105.3 g at 1.11 g/mL is __________ mL.
Answer: 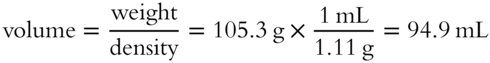
![]() A liter of milk weighs 946 grams. What is the density of the milk in g/mL (to the nearest thousandth)? __________
A liter of milk weighs 946 grams. What is the density of the milk in g/mL (to the nearest thousandth)? __________
Answer: 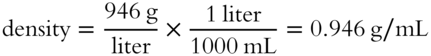
![]() Instead of using weight percentage of solute in the solution and converting to volume, it is possible to determine volume percentage of the solute in the solution. Volume percentage is most often used when dealing with a solution involving two liquids, such as alcohol and water. Finding the volume percentage of the solute involves the familiar percentage formula.
Instead of using weight percentage of solute in the solution and converting to volume, it is possible to determine volume percentage of the solute in the solution. Volume percentage is most often used when dealing with a solution involving two liquids, such as alcohol and water. Finding the volume percentage of the solute involves the familiar percentage formula.
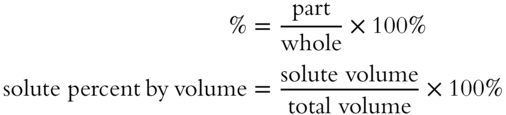
A certain brand of homogenized milk contains 35 mL of butterfat in a total volume of 1 liter. What is the percentage by volume of butterfat? __________
Answer: solute percent by volume
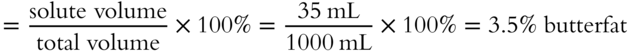
![]() The drugstore sells a solution of 3% hydrogen peroxide (H2O2) by volume. In a 150 mL quantity of this solution, there are how many milliliters of H2O2? (Hint: The volume of the solute is the unknown.) __________
The drugstore sells a solution of 3% hydrogen peroxide (H2O2) by volume. In a 150 mL quantity of this solution, there are how many milliliters of H2O2? (Hint: The volume of the solute is the unknown.) __________
Answer: 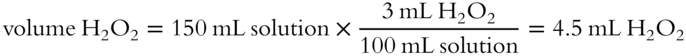
MOLARITY
![]() Instead of the percentage by weight or volume of a solute within the solution, we may wish to deal with moles of solute within the solution. Molarity describes the moles of a solute within a total solution of 1 liter. A solution that is 0.3 molar means that 0.3 mol of solute are contained in 1 liter of solution. One liter of 0.5 molar NaCl solution contains how many moles of NaCl? __________
Instead of the percentage by weight or volume of a solute within the solution, we may wish to deal with moles of solute within the solution. Molarity describes the moles of a solute within a total solution of 1 liter. A solution that is 0.3 molar means that 0.3 mol of solute are contained in 1 liter of solution. One liter of 0.5 molar NaCl solution contains how many moles of NaCl? __________
Answer: 0.5
![]() Molarity (abbreviated by the italicized capital letter M) indicates the moles of solute contained within 1 liter of solution. If 3 mol of sugar were dissolved in enough water to make a total solution of 1 liter, the solution would be _____ M sugar.
Molarity (abbreviated by the italicized capital letter M) indicates the moles of solute contained within 1 liter of solution. If 3 mol of sugar were dissolved in enough water to make a total solution of 1 liter, the solution would be _____ M sugar.
Answer: 3
![]() Again, molarity indicates moles of solute per liter of solution. The molarity specifies the concentration of solute within the solution. All of the 3 M sugar solution from frame 46 is a 3 M sugar solution, even the last few drops, because molarity is the concentration of the solute. If you took 1 liter of 3 M sugar solution and poured half of it (500 mL) into another container, what would be the molarity (concentration) of sugar solution in each container? __________
Again, molarity indicates moles of solute per liter of solution. The molarity specifies the concentration of solute within the solution. All of the 3 M sugar solution from frame 46 is a 3 M sugar solution, even the last few drops, because molarity is the concentration of the solute. If you took 1 liter of 3 M sugar solution and poured half of it (500 mL) into another container, what would be the molarity (concentration) of sugar solution in each container? __________
Answer: 3 M (The concentration of sugar solute remains the same—3 mol/liter.)
![]() We could also say that each container had 1½ mol per half liter concentration. It is easier to use a standard such as moles per liter, however. An analogy is miles per hour as a designation for speed. If you were driving at a steady 50 mph for half an hour, you would expect to have driven 25 miles. You were also driving at a speed of 25 miles per half hour, but it is more convenient to use miles per hour as a standard for speed rather than miles per half hour. If you took 1½ mol of sugar and added enough water to make a total of 500 mL of solution, the concentration of sugar solute would be ______M. (Remember molarity = moles per liter.)
We could also say that each container had 1½ mol per half liter concentration. It is easier to use a standard such as moles per liter, however. An analogy is miles per hour as a designation for speed. If you were driving at a steady 50 mph for half an hour, you would expect to have driven 25 miles. You were also driving at a speed of 25 miles per half hour, but it is more convenient to use miles per hour as a standard for speed rather than miles per half hour. If you took 1½ mol of sugar and added enough water to make a total of 500 mL of solution, the concentration of sugar solute would be ______M. (Remember molarity = moles per liter.)
Answer: 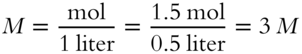
![]() If 0.1 mol of NaCl was dissolved in water to make 100 mL of solution, the concentration of NaCl in the solution will be __________ M.
If 0.1 mol of NaCl was dissolved in water to make 100 mL of solution, the concentration of NaCl in the solution will be __________ M.
Answer: 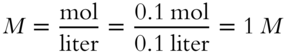
![]() The weight of a solute can be changed to moles to determine the molarity of a solution.
The weight of a solute can be changed to moles to determine the molarity of a solution.
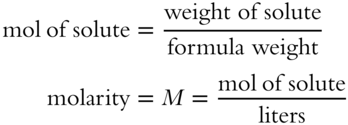
The two formulas can be combined as follows:
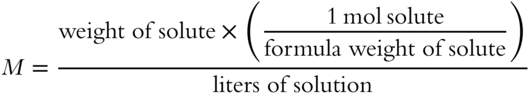
The formula weight for NaCl is 58.4 grams. (To review, see Chapter 4.) If 87.8 grams of NaCl solute is dissolved in enough water to make 3 liters of solution, what molarity would the resulting NaCl solution have?
M = __________
Answer: 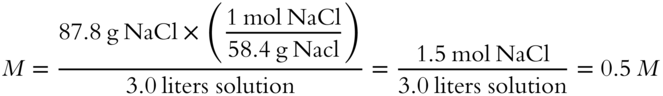
![]() The molarity of a 1 liter solution containing 37.5 grams of Ba(MnO4)2 is _______M.
The molarity of a 1 liter solution containing 37.5 grams of Ba(MnO4)2 is _______M.
Answer: 
![]() The formula weight of NaCl is 58.4 g/mol. How many grams of NaCl are needed to make 2 liters of a 0.3 M NaCl solution? __________
The formula weight of NaCl is 58.4 g/mol. How many grams of NaCl are needed to make 2 liters of a 0.3 M NaCl solution? __________
Answer: 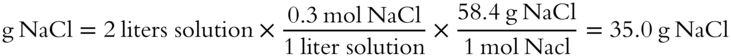
![]() In aqueous solution, a strong electrolyte such as NaCl dissociates completely (separates into Na+ and Cl− ions). A solution that is 0.3 M NaCl solution is actually made up of 0.3 M Na+ ion and 0.3 M Cl− ion.
In aqueous solution, a strong electrolyte such as NaCl dissociates completely (separates into Na+ and Cl− ions). A solution that is 0.3 M NaCl solution is actually made up of 0.3 M Na+ ion and 0.3 M Cl− ion.
A 0.2 M solution of HCl (which is a strong electrolyte) would be made up of __________ M H+ ion and __________ M Cl− ion.
Answer: 0.2; 0.2
![]() H2SO4 is also a strong electrolyte that dissociates into two H+ ions and one SO42− ion.
H2SO4 is also a strong electrolyte that dissociates into two H+ ions and one SO42− ion.
![]()
That is, one molecule of H2SO4 yields two H+ ions and one SO42− ion in aqueous solution. One mole of H2SO4 molecules would yield 2 mol of H+ ions and 1 mol of SO42− ions. Two moles of H2SO4 would yield 4 mol H+ ions, and so on. So a 0.1 M solution of H2SO4 results in 0.2 M H+ ions and 0.1 M SO42− ions.
Assume that H3PO4 is a strong electrolyte and dissociates completely.
![]()
A solution of H3PO4 that is 0.1 M results in __________ M H+ ions and __________ M PO43− ions.
Answer: 0.3; 0.1
![]() A liter of 0.1 M solution of H3PO4 contains how many times the concentration of hydrogen ions as does a liter of 0.1 M solution of HCl? __________
A liter of 0.1 M solution of H3PO4 contains how many times the concentration of hydrogen ions as does a liter of 0.1 M solution of HCl? __________
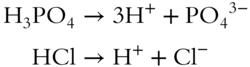
Answer: three times as much
![]() A liter of 0.1 M solution of NaOH, which is a strong electrolyte, produces a _____M concentration of OH− ions and a _____M concentration of Na+ ions.
A liter of 0.1 M solution of NaOH, which is a strong electrolyte, produces a _____M concentration of OH− ions and a _____M concentration of Na+ ions.
Answer: 0.1; 0.1
![]() What would be the OH− ion concentration in a 0.1 M Ba(OH)2 solution? Assume Ba(OH)2 is a strong electrolyte. __________
What would be the OH− ion concentration in a 0.1 M Ba(OH)2 solution? Assume Ba(OH)2 is a strong electrolyte. __________
Answer: 0.2 M OH−
We will use H+ ion and OH− ion molar concentrations later in Chapter 13, when we further discuss acids and bases.
COLLIGATIVE PROPERTIES OF SOLUTIONS AND MOLALITY
![]() The physical properties of a solution are somewhat different from the properties of the pure solvent. The amount and kind of solute in the solution affects such properties as boiling point, freezing point, and vapor pressure. These properties that vary according to the ratio of the weights of the solute and solvent are known as colligative properties.
The physical properties of a solution are somewhat different from the properties of the pure solvent. The amount and kind of solute in the solution affects such properties as boiling point, freezing point, and vapor pressure. These properties that vary according to the ratio of the weights of the solute and solvent are known as colligative properties.
In general, as solute is added the resulting solution will have a lower freezing point, lower vapor pressure, and higher boiling point than the pure solvent.
What happens to the freezing point of the water in an automobile cooling system when radiator antifreeze (the solute) is added to water (the solvent)? _____________
Answer: The freezing point decreases or lowers (The typical automobile radiator antifreeze solution is made up of half water as the solvent and half ethylene glycol as the solute. The freezing point is typically about −37°Celsius. The boiling point of the solution is increased.)
![]() When dealing with solutions for chemical reactions such as neutralization, it is practical to use molarity (M) as the standard for the concentration of the solute in the solution. Molarity indicates moles of solute within each liter of solution. When working with colligative properties, however, it is more useful to deal with a different standard of the concentration of solute in the solution. This standard is molality. Molality, abbreviated by the small letter m, indicates the moles of solute in each kilogram of solvent.
When dealing with solutions for chemical reactions such as neutralization, it is practical to use molarity (M) as the standard for the concentration of the solute in the solution. Molarity indicates moles of solute within each liter of solution. When working with colligative properties, however, it is more useful to deal with a different standard of the concentration of solute in the solution. This standard is molality. Molality, abbreviated by the small letter m, indicates the moles of solute in each kilogram of solvent.
When dealing with physical properties such as the boiling point elevation of a solution, it is more practical to use moles of solute per (liter of solution, kilogram of solvent) __________
Answer: kilogram of solvent
![]() Molarity is abbreviated by __________ and indicates moles of solute per __________ of ________. Molality is abbreviated by __________ and indicates moles of solute per ________ of __________
Molarity is abbreviated by __________ and indicates moles of solute per __________ of ________. Molality is abbreviated by __________ and indicates moles of solute per ________ of __________
Answer: M; liter; solution; m; kilogram; solvent
![]() Here is the equation for determining molality.
Here is the equation for determining molality.
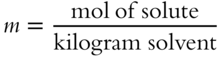
Three moles of sugar are added to 1000 grams of H2O solvent. The molality of the resulting solution is _______ m. (Hint: Change weight of solvent to kilograms, abbreviated kg.)
Answer: 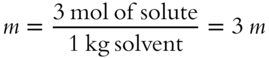
![]() Two moles of sugar are added to 500 grams of H2O solvent. The molality of the resulting solution is _______ m.
Two moles of sugar are added to 500 grams of H2O solvent. The molality of the resulting solution is _______ m.
Answer: 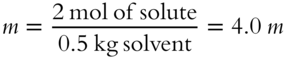
![]() Molality can also be determined from the number of grams of solute by substituting the formula for moles in the formula for molality.
Molality can also be determined from the number of grams of solute by substituting the formula for moles in the formula for molality.

The new formula becomes:
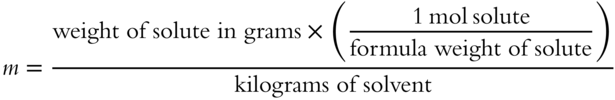
Let's use this formula to determine the molality of a glucose solution. Fifty grams of glucose (C6H1206) are added to 0.250 kg of water. Assume the formula weight of glucose (C6H1206) is 180 grams. The molality of this glucose solution is _________ m.
Answer: 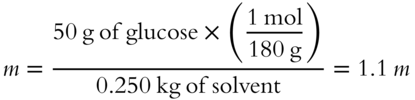
![]() Determine the molality of a solution involving 22.5 grams of glucose dissolved in 500 grams of water. The formula weight of glucose is 180 grams. The molality of this glucose solution (to the nearest hundredth) is_______ m. (Note: Be sure to change grams of solvent to kilograms.)
Determine the molality of a solution involving 22.5 grams of glucose dissolved in 500 grams of water. The formula weight of glucose is 180 grams. The molality of this glucose solution (to the nearest hundredth) is_______ m. (Note: Be sure to change grams of solvent to kilograms.)
Answer: 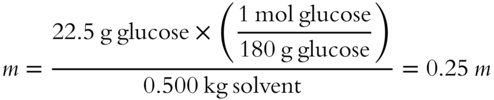
![]() Determine the molality of a solution involving 116.8 grams of NaCl dissolved in 500 grams of water. The molality of this NaCl solution is m.
Determine the molality of a solution involving 116.8 grams of NaCl dissolved in 500 grams of water. The molality of this NaCl solution is m.
Answer: 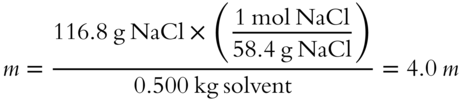
![]() How many grams of solute are necessary to produce a 2.50 m solution of CaCl2 dissolved in 500 grams of water? The formula weight of CaCl2 is 111 grams.
How many grams of solute are necessary to produce a 2.50 m solution of CaCl2 dissolved in 500 grams of water? The formula weight of CaCl2 is 111 grams.
Answer: 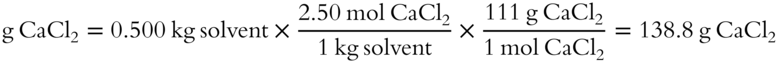
![]() In dealing with liquid solutes in liquid solvents, the amount of solute and sometimes the solvent is given in terms of volumes such as liters or milliliters. Volumes must be converted to weights in order to determine the molality of the resulting solution. If we are given the volume of a liquid, we also need the density of the liquid in order to determine its weight. Density is usually expressed as grams per cubic centimeter or grams per milliliter. To find the weight in grams of a liquid, multiply the density times the volume (density × volume = weight). The density of ethyl alcohol is found to be 0.80 g/mL. Determine the weight in grams of 23.75 mL of ethyl alcohol. __________
In dealing with liquid solutes in liquid solvents, the amount of solute and sometimes the solvent is given in terms of volumes such as liters or milliliters. Volumes must be converted to weights in order to determine the molality of the resulting solution. If we are given the volume of a liquid, we also need the density of the liquid in order to determine its weight. Density is usually expressed as grams per cubic centimeter or grams per milliliter. To find the weight in grams of a liquid, multiply the density times the volume (density × volume = weight). The density of ethyl alcohol is found to be 0.80 g/mL. Determine the weight in grams of 23.75 mL of ethyl alcohol. __________
Answer: weight of alcohol = 0.80 g/mL × 23.75 mL = 19.0 grams of alcohol
![]() Determine the weight in grams of 300 mL of benzene (C6H6) if the density of benzene is 0.90 g/mL. __________
Determine the weight in grams of 300 mL of benzene (C6H6) if the density of benzene is 0.90 g/mL. __________
Answer: weight of benzene = 0.90 g/mL × 300 mL = 270 grams of benzene
![]() A solution is made up of 23.75 mL of ethyl alcohol solute added to 300 mL of benzene solvent. In frames 67 and 68 you have just determined the weight of the ethyl alcohol to be 19.0 grams and the weight of benzene to be 270 grams. The formula weight of C2H5OH (ethyl alcohol) is 46 grams. Determine the molality of this solution.
A solution is made up of 23.75 mL of ethyl alcohol solute added to 300 mL of benzene solvent. In frames 67 and 68 you have just determined the weight of the ethyl alcohol to be 19.0 grams and the weight of benzene to be 270 grams. The formula weight of C2H5OH (ethyl alcohol) is 46 grams. Determine the molality of this solution.
Answer: 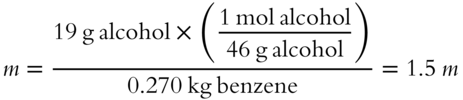
MOLE FRACTION AND VAPOR PRESSURE
![]() Placing a solute in a solvent lowers the vapor pressure of the solvent. If both the solvent and the solute are liquids, the vapor pressure of the solution is due to both solute and solvent. The amount contributed to the vapor pressure by either solute or solvent depends upon the mole fraction of each within the solution.
Placing a solute in a solvent lowers the vapor pressure of the solvent. If both the solvent and the solute are liquids, the vapor pressure of the solution is due to both solute and solvent. The amount contributed to the vapor pressure by either solute or solvent depends upon the mole fraction of each within the solution.
![]()
The mole fraction is simply the number of moles of one of the components of the solution (either solute or solvent) divided by the total number of moles in the solution.
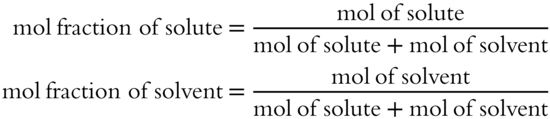
A solution contains 3 mol of solute and 12 mol of solvent. What is the mole fraction of solute? _______________?
Answer: 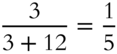 or 0.2
or 0.2
![]() A solution contains 3 mol of solute and 12 mol of solvent. What is the mole fraction of solute? _______
A solution contains 3 mol of solute and 12 mol of solvent. What is the mole fraction of solute? _______
Answer: 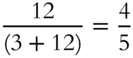 or 0.8
or 0.8
![]() All mole fractions in a solution must add up to 1. A solution is made up of 23 grams of ethyl alcohol (C2H5OH) and 156 grams of benzene (C6H6). The formula weight of C2H5OH is 46 g/mol. The formula weight of C6H6 is 78 g/mol.
All mole fractions in a solution must add up to 1. A solution is made up of 23 grams of ethyl alcohol (C2H5OH) and 156 grams of benzene (C6H6). The formula weight of C2H5OH is 46 g/mol. The formula weight of C6H6 is 78 g/mol.
1. What is the mole fraction of ethyl alcohol? __________
2. What is the mole fraction of benzene? __________
3. What is the sum of the mole fractions of ethyl alcohol and benzene? ________
Answer:
1. 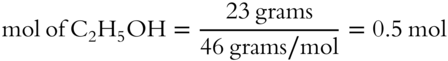
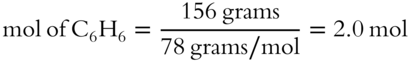
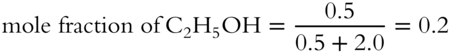
2. 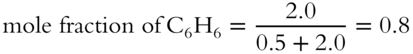
3. The combined mole fractions are equal to 1 (0.2 + 0.8 = 1).
![]() A solution can be made of more than two components. To determine the mole fraction of one component in a solution made up of several components, just divide the moles of the component by the total moles in the solution.
A solution can be made of more than two components. To determine the mole fraction of one component in a solution made up of several components, just divide the moles of the component by the total moles in the solution.
In a solution made up of A, B, and C, suppose you wish to know the mole fraction of A.
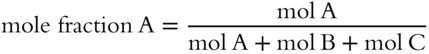
In this same solution, what is the mole fraction of B? __________
Answer: 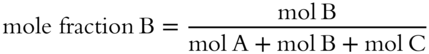
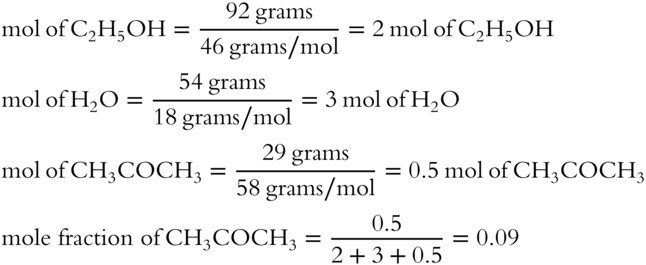
![]() A solution is made up of 92 grams of ethyl alcohol (C2H5OH), 54 grams of H2O, and 29 grams of acetone (CH3COCH3). The formula weight of C2H5OH is 46 grams. The formula weight of H2O is 18 grams. The formula weight of CH3COCH3 is 58 grams. Determine the mole fraction of acetone in the solution (to the nearest hundredth). __________
A solution is made up of 92 grams of ethyl alcohol (C2H5OH), 54 grams of H2O, and 29 grams of acetone (CH3COCH3). The formula weight of C2H5OH is 46 grams. The formula weight of H2O is 18 grams. The formula weight of CH3COCH3 is 58 grams. Determine the mole fraction of acetone in the solution (to the nearest hundredth). __________
Answer:
![]() A solution made of two or more liquids has a vapor pressure that is determined by the mole fractions of the liquids that make up the solution. This general statement leads to Raoult's Law. Raoult's Law is expressed mathematically as:
A solution made of two or more liquids has a vapor pressure that is determined by the mole fractions of the liquids that make up the solution. This general statement leads to Raoult's Law. Raoult's Law is expressed mathematically as:
![]()
PA is the vapor pressure contributed by liquid A in the solution. XA is the mole fraction of liquid A in the solution. ![]() is the vapor pressure of liquid A in its pure form.
is the vapor pressure of liquid A in its pure form.
A solution is made up of toluene and benzene. Toluene is present in a mole fraction of 0.6 and has a vapor pressure of 70 torr in the pure form. We want to find the vapor pressure contributed by toluene in the solution.
![]()
That is, the vapor pressure contributed by toluene equals the mole fraction of toluene multiplied by the vapor pressure of pure toluene. The vapor pressure contributed by toluene = __________ torr.
Answer:
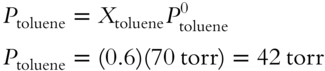
![]() In the same solution of benzene and toluene, benzene also contributes to the vapor pressure of the solution.
In the same solution of benzene and toluene, benzene also contributes to the vapor pressure of the solution.
![]()
To determine the vapor pressure contributed by benzene, we must know the mole fraction of benzene in the solution and the vapor pressure of pure benzene. There are only two liquids in the solution. All mole fractions in a solution add up to equal 1. If toluene accounts for a mole fraction of 0.6, what is the benzene mole fraction? _______________
Answer: 0.4 (1 — 0.6)
![]() In the solution of toluene and benzene, the benzene has a mole fraction of 0.4 and the vapor pressure of benzene in pure form is equal to 190 torr. Use Raoult's Law to calculate the vapor pressure contributed by benzene in this solution. __________
In the solution of toluene and benzene, the benzene has a mole fraction of 0.4 and the vapor pressure of benzene in pure form is equal to 190 torr. Use Raoult's Law to calculate the vapor pressure contributed by benzene in this solution. __________
Answer:
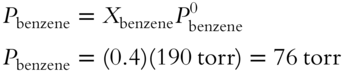
![]() In our solution made up of toluene and benzene, toluene contributes 42 torr to the vapor pressure of the solution and benzene contributes 76 torr to the vapor pressure of the solution. The total vapor pressure above the solution is the sum of all of the partial vapor pressures (from Dalton's Law of partial pressures, discussed in Chapter 8). The total vapor pressure of the solution is __________ torr.
In our solution made up of toluene and benzene, toluene contributes 42 torr to the vapor pressure of the solution and benzene contributes 76 torr to the vapor pressure of the solution. The total vapor pressure above the solution is the sum of all of the partial vapor pressures (from Dalton's Law of partial pressures, discussed in Chapter 8). The total vapor pressure of the solution is __________ torr.
Answer: 118 (42 + 76)
![]() Raoult's Law predicts ideal vapor pressures. The actual vapor pressure may be slightly more or less than predicted. Another toluene and benzene solution is prepared with a mole fraction of 0.7 for toluene. Assume the vapor pressure of pure toluene liquid to be 70 torr and the vapor pressure of pure benzene liquid to be 190 torr.
Raoult's Law predicts ideal vapor pressures. The actual vapor pressure may be slightly more or less than predicted. Another toluene and benzene solution is prepared with a mole fraction of 0.7 for toluene. Assume the vapor pressure of pure toluene liquid to be 70 torr and the vapor pressure of pure benzene liquid to be 190 torr.
1. What is the ideal vapor pressure contributed by toluene in the solution? __________
2. What is the total ideal vapor pressure of this solution? __________
Answer:
1. ![]()
![]()
2. The mole fractions of all components in the solution add up to 1. Since toluene accounts for 0.7 mol fraction, the remainder must be attributed to benzene.
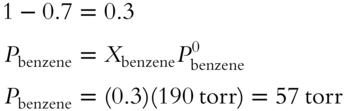
The total vapor pressure is the sum of all partial vapor pressures in the solution. The total vapor pressure is 49 torr + 57 torr = 106 torr.
![]() Raoult's Law also applies to solutions in which the solute is a solid with no vapor pressure. If solid sugar is dissolved in water, the vapor pressure of the water is lowered according to its mole fraction as predicted by Raoult's Law. The solid sugar had almost no measurable vapor pressure, so the entire vapor pressure of the sugar-water solution is contributed by the water. The vapor pressure of pure water is 23.8 torr at 25°C. Sugar is added to the water until the water makes up a mole fraction of 0.75 in the solution.
Raoult's Law also applies to solutions in which the solute is a solid with no vapor pressure. If solid sugar is dissolved in water, the vapor pressure of the water is lowered according to its mole fraction as predicted by Raoult's Law. The solid sugar had almost no measurable vapor pressure, so the entire vapor pressure of the sugar-water solution is contributed by the water. The vapor pressure of pure water is 23.8 torr at 25°C. Sugar is added to the water until the water makes up a mole fraction of 0.75 in the solution.
1. What is the vapor pressure of the water in the solution? __________
2. Since the sugar has no measurable vapor pressure, what is the total vapor pressure of the solution? __________
Answer:
1. ![]()
![]()
2. The total vapor pressure of the solution is the same as the vapor pressure contributed by the water since the sugar has no vapor pressure. Total vapor pressure = 17.9 torr.
BOILING POINT ELEVATION
![]() The vapor pressure of a solvent (such as water) is reduced when a solute with no vapor pressure of its own is added to the solvent. Remember that the boiling point of a liquid is the temperature at which the vapor pressure equals the confining pressure. If a nonvolatile solute (one with no vapor pressure of its own) is added to a solvent, the boiling point of the solvent is raised because the vapor pressure is reduced. The more solute added, the more the vapor pressure is reduced, and the more the boiling point is increased.
The vapor pressure of a solvent (such as water) is reduced when a solute with no vapor pressure of its own is added to the solvent. Remember that the boiling point of a liquid is the temperature at which the vapor pressure equals the confining pressure. If a nonvolatile solute (one with no vapor pressure of its own) is added to a solvent, the boiling point of the solvent is raised because the vapor pressure is reduced. The more solute added, the more the vapor pressure is reduced, and the more the boiling point is increased.
Adding 1 mol of a nonvolatile solute that is also a nonelectrolyte to 1 kg of water raises the boiling point by 0.51°C. If the boiling point of pure water is 100.00°C, then adding 1 mol of nonvolatile and nonelectrolytic solute to 1 kg of water raises the boiling point of the solution to the new temperature of __________°C.
Answer: 100.51 (100.00°+ 0.51°C)
![]() The presence of 1 mol of any nonvolatile nonelectrolyte such as sugar added to 1 kg of water raises the boiling point of the water by 0.51°C. This 0.51°C is known as the molal boiling point constant for water.
The presence of 1 mol of any nonvolatile nonelectrolyte such as sugar added to 1 kg of water raises the boiling point of the water by 0.51°C. This 0.51°C is known as the molal boiling point constant for water.
A sugar-water solution that is 1 m boils at 100.51°C at standard pressure. A sugar-water solution of 1 m raises the boiling point of water by 0.51°C. A sugar-water solution of 3 m raises the boiling point by three times the molal boiling point constant (3 × 0.51 = 1.53°C) and raises the boiling point of the solution to what new temperature?_____ °C
Answer: 101.53°C [(3 × 0.51) + 100 = 101.53]
![]() The molal boiling point constant is usually abbreviated as Kb. The Kb for water is 0.51°C/m. (The Kb varies for other solvents.) A useful formula for predicting the boiling point of solutions is:
The molal boiling point constant is usually abbreviated as Kb. The Kb for water is 0.51°C/m. (The Kb varies for other solvents.) A useful formula for predicting the boiling point of solutions is:
![]()
The increase in the boiling point (ΔTb) equals the molal boiling point constant (Kb) multiplied by the molality (m) of the solution. For example, if the molality of a sugar-water solution is 4 m, then the increase in boiling point (ΔTb) equals 0.51°C multiplied by 4, or 2.04°C. Calculate the increase in the boiling point if a sugar-water solution has a molality of 5 m.
ΔTb = __________
Answer: Kb × m = 0.51°C × 5 = 2.55°C
![]() A sugar-water solution of 7.0 m would increase the boiling point by __________°C.
A sugar-water solution of 7.0 m would increase the boiling point by __________°C.
Answer: 7.0 × 0.51°C = 3.57°C
![]() We can also modify the formula to determine the molality of a solution, by solving for m.
We can also modify the formula to determine the molality of a solution, by solving for m.
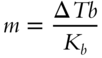
What is the molality of a sugar-water solution that increases the boiling point by 0.255°C? __________
Answer: 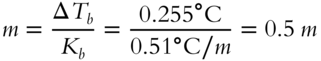
FREEZING POINT DEPRESSION
![]() A nonvolatile solute not only raises the boiling point of the solution, but it also lowers the freezing point of the solution. Each mole of any nonvolatile nonelectrolyte, such as sugar, in 1 kg of water will lower the freezing point by 1.86°C. Since the normal freezing point of water is 0°C, the new freezing point of a solution of 1 mol of sugar added to 1 kg of water will be __________°C.
A nonvolatile solute not only raises the boiling point of the solution, but it also lowers the freezing point of the solution. Each mole of any nonvolatile nonelectrolyte, such as sugar, in 1 kg of water will lower the freezing point by 1.86°C. Since the normal freezing point of water is 0°C, the new freezing point of a solution of 1 mol of sugar added to 1 kg of water will be __________°C.
Answer: −1.86
![]() The molal freezing point constant for water is −1.86°C/m. The molal freezing point constant is abbreviated as Kf and varies for different solvents as does Kb. The formula for determining the freezing point decrease is:
The molal freezing point constant for water is −1.86°C/m. The molal freezing point constant is abbreviated as Kf and varies for different solvents as does Kb. The formula for determining the freezing point decrease is:
![]()
Determine the value of ΔTf (the amount of decrease in the freezing point) of a 3 m sugar-water solution. ΔTf = __________
Answer: −1.86°C × 3 = −5.58°C
![]() The freezing point change for a 2.5 m solution of sugar-water would be __________°C.
The freezing point change for a 2.5 m solution of sugar-water would be __________°C.
Answer: −4.65 (−1.86°C × 2.5 = −4.65°C)
![]() As with the formula for boiling point increase, the freezing point decrease formula can also be used to determine the molality of a solution.
As with the formula for boiling point increase, the freezing point decrease formula can also be used to determine the molality of a solution.
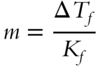
The molality of a sugar-water solution that freezes at −3.50°C (to the nearest hundredth) is __________
Answer: The change in freezing point is −3.50°C.
![]() The sugar solution has been used in the previous examples because it is a nonelectrolyte and the formulas are valid only for such substances. The formulas involving the molal freezing and boiling point constants are valid for which of the following?
The sugar solution has been used in the previous examples because it is a nonelectrolyte and the formulas are valid only for such substances. The formulas involving the molal freezing and boiling point constants are valid for which of the following?
· _______ (a) NaCl, a strong electrolyte
· _______ (b) sugar, a nonelectrolyte
· _______ (c) acetic acid, a weak electrolyte
Answer: only (b)
![]() Every mole of a nonelectrolyte will lower the freezing point and raise the boiling point by an amount equal to Kf and Kb. Each mole has a specific number of particles, so we can say that the greater the number of solute particles in a solvent the greater will be the freezing point decrease and boiling point increase. A strong electrolyte such as 1 mol of NaCl in a kilogram of water is actually a solution of 1 mol of Na+ ions and 1 mol of Cl− ions, or 2 mol of particles. So a solution of 1 m NaCl would have the same effect as a 2 m solution of sugar. What would be the change in freezing point of a 1 m NaCl solution? __________
Every mole of a nonelectrolyte will lower the freezing point and raise the boiling point by an amount equal to Kf and Kb. Each mole has a specific number of particles, so we can say that the greater the number of solute particles in a solvent the greater will be the freezing point decrease and boiling point increase. A strong electrolyte such as 1 mol of NaCl in a kilogram of water is actually a solution of 1 mol of Na+ ions and 1 mol of Cl− ions, or 2 mol of particles. So a solution of 1 m NaCl would have the same effect as a 2 m solution of sugar. What would be the change in freezing point of a 1 m NaCl solution? __________
Answer: −1.86°C × 2 = −3.72°C
![]() K2SO4 is a strong electrolyte.
K2SO4 is a strong electrolyte.
![]()
Calculate the change in the boiling point of a 1 m K2SO4 solution. __________
Answer: 0.51°C × 3 = 1.53°C
![]() A 1 m aqueous solution of carbonic acid, H2CO3, has lowered the freezing point −3.00°C. Is H2CO3 a strong electrolyte, weak electrolyte, or nonelectrolyte? ______________
A 1 m aqueous solution of carbonic acid, H2CO3, has lowered the freezing point −3.00°C. Is H2CO3 a strong electrolyte, weak electrolyte, or nonelectrolyte? ______________
Answer: weak electrolyte (If it were a nonelectrolyte the freezing point would be lowered −1.86 × 1 = −1.86°C. If it were a strong electrolyte and dissociated as H2CO3 → H+ + HCO3−, the lowering would be −1.86 × 2 = −3.72°C. It might also dissociate as H2CO3 → 2H+ + CO32−, giving a lowering of −1.86 × 3 = −5.58°C. The ΔTf falls between the expected values for a nonelectrolyte and strong electrolyte and is, therefore, presumed to be a weak electrolyte.)
In reality, 1 m NaCl and 1 m K2SO4 do not affect the freezing point and boiling point changes as expected. They do not have two or three times the effect predicted by their 100% dissociation. They have somewhat less effect because the “activity” of the ions is not ideal, but that activity is beyond the scope of this book. In general, nonelectrolytes conform to the expected molal freezing and boiling point constants. Weak electrolytes decrease the freezing point and increase the boiling point only slightly beyond that of nonelectrolytes. Strong electrolytes (which dissociate completely) come close to having each ion act independently in increasing the boiling point and decreasing the freezing points.
You have just learned several things about solutions that chemists and industries use daily in the laboratory or in the factory to produce desired products or results. We could not operate without understanding the solute-solvent properties and relationships we have discussed. You will encounter the concentration terms in the remaining chapters of this book and most certainly in any other chemistry course you might take.
SELF-TEST
This self-test is designed to show how well you have mastered this chapter's objectives. Correct answers and review instructions follow the test. Round answers to the nearest hundredth unless otherwise indicated.
1. Complete and balance the following equations. Indicate if the product(s) of each equation is called an acid or a base.
1. CO2 + H2O → ___________
2. Na2O + H2O → ___________
2. Complete and balance the following equations. Indicate if the product(s) of each equation is called an acid or a base.
1. Li2O + H2O → _________________________
2. P2O5 + 3H2O → _________________________
3. How many grams of H2O could you obtain from 1 kg of CuSO4 · 5H2O (to the nearest gram)? ______________
4. The blue hydrate CuSO4 . ?H2O is heated to give:
![]()
A sample of the hydrate weighed 499.36 g before heating. After heating, the anhydrous CuSO4 weighed 319.22 g. Determine:
1. the weight of the water formed. ______________________
2. the moles of water formed. ______________________
3. the moles of CuSO4 formed. ______________________
4. the true hydrate formula. ______________________
5. Ammonia gas is passed into water yielding a solution whose density is 0.93 g/mL and is 18.6% by weight of NH3. What is the weight of NH3 per milliliter of solution?
6. The grocery store sells a solution of vinegar that is 5.0% acetic acid (HC2H3O2) by volume. In a 250-milliliter quantity of this solution, there are how many milliliters of HC2H3O2? (Hint: The volume of the solute is the unknown.) _________________
7. What is the molarity of a solution that contains 16.5 grams (NH4)2SO4 in 100 mL of solution? __________
8. The formula weight of NaCl is 58.4 g/mol. How many grams of NaCl are needed to make 4 liters of 0.3 M NaCl solution?
9. Determine the molality of a solution involving 18.6 grams of glucose dissolved in 750 grams of water. The formula weight of glucose is 180 grams. The molality of this glucose solution (to the nearest hundredth) is _____m.
10. Determine the molality of a solution involving 46.3 grams of NaCl dissolved in 845 grams of water. The formula weight of NaCl is 58.4 grams. The molality of this NaCl solution (to the nearest hundredth) is _____m.
11. A solution contains 58 grams of acetone (CH3COCH3), 69 grams of ethyl alcohol (C2H5OH), and 63 grams of H2O. What is the mole fraction of water in this mixture? ____________________
12. A solution contains 238.5 g of glucose (C6H12O6) and 485.5 g of water. What is the mole fraction of glucose in this mixture?
13. What is the ideal vapor pressure contributed by benzene in a toluene-benzene solution prepared with a mole fraction of 0.8 for benzene? Assume pure toluene to be 0.092 atm and the vapor pressure of pure benzene to be 0.250 atm.
14. A solution containing 15 grams of an unknown substance dissolved in 100 grams of water has a boiling point of 100.255°C. What is the molecular weight of the unknown (to the nearest gram)? __________
15. A 1 m solution of HCN has a boiling point of 100.72°C. Is it a strong electrolyte, a weak electrolyte, or nonelectrolyte? Defend your answer. _________________________________
ANSWERS
Compare your answers to the self-test with those given below. If you answer all questions correctly, you are ready to proceed to the next chapter. If you miss any, review the frames indicated in parentheses following the answers. If you miss several questions, you should probably reread the chapter carefully.
1. H2CO3 (acid); (b) 2NaOH (base) (frames 5—11)
2. (a) 2LiOH (base) (b) 2H3PO4 (acid) [frames 5-11]
3.
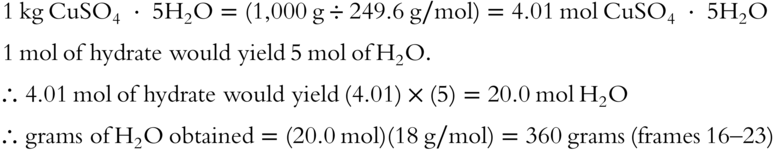
4.
1. wt. of H2O = 499.36 — 319.22 g = 180.18 g H2O
2. mol. H2O = 180.18 g x (1 mol. H2O/18.0 g H2O) = 10 mol H2O
3. mol. CuSO4 = 319.22 g x (1 mol. CuSO4 / 169.61 g = 2 mol CuSO4
4. mol H2O / mol CuSO4 = 10/2 = 5
The formula is CuSO4 . 5H2O. [frames 24-28]
5. weight NH3 = density × % by weight × volume = 0.93 g/mL × 0.186 × 1 mL = 0.17 grams (frames 34—41)
6. volume HC2H3O2 = 250 mL solution x (5.0 mL HC2H3O2 / 100 mL solution) = 12.5 mL HC2H3O2 [frames 34-44]
7.
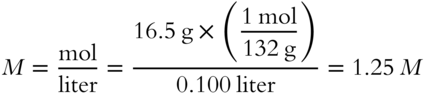
(frames 45—51)
8. g NaCl = 4 liters of solution x (0.3 mol NaCl / 1 L solution) x (58.4 g NaCl / 1 mol NaCl)= 70.1 g NaCl [frames 45-52]
9. First obtain moles of glucose.
18.6 glucose 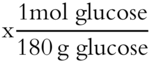 = 0.103 moles glucose
= 0.103 moles glucose
Next, solve for molality.
molality (m) = moles of solute / kg of solvent = 0.103 mol glucose / 0.750 kg = 0.137 m [frames 58-69]
10. First obtain moles of NaCl.
46.3 g NaCl 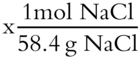 = 0.793 moles NaCl
= 0.793 moles NaCl
Next, solve for molality.
molality (m) = moles of solute / kg of solvent = 0.793 mol NaCl / 0.845 kg = 0.938 m [frames 58-69]
11.
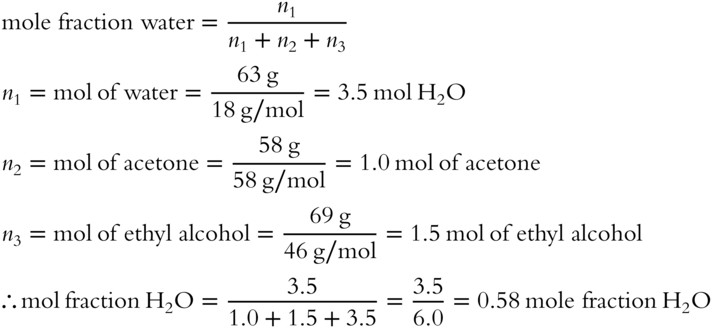
(frames 70—74)
12. 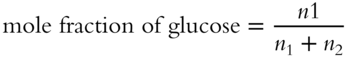
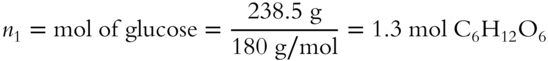
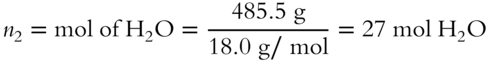
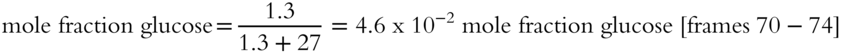
13. Pbenzene = XbenzenePobenzene = (0.8)(0.250 atm) = 0.200 atm [frames75-79]
14.
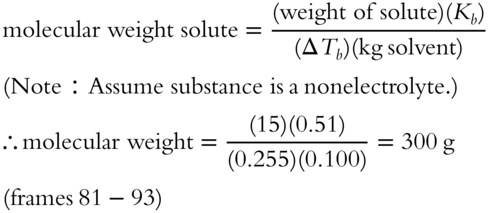
15. If it is nonelectrolyte, 1 m HCN should boil at 100.51° C (100° C + ΔTb).
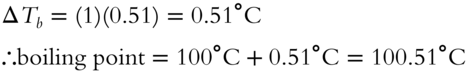
If it is a strong electrolyte (HCN → H+ + CN−), then a 1 m solution should boil at 101.02°C.
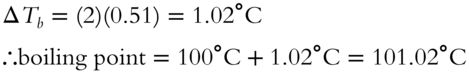
Since the observed boiling point is between these two extremes, we would conclude that it is a weak electrolyte, only partially dissociated. (frames 81−93)