Chemistry: A Self-Teaching Guide - Post R., Snyder C., Houk C.C. 2020
Chemical Equilibrium
You have just learned several properties of solutions (mixtures of solids, liquids, and gases). We have discussed reactions that go to completion (reactants totally consumed, leaving only new products) in Chapter 5 and electrolytes that dissociate completely in water in Chapter 11. Both of these concepts imply a one-way reaction, continuous movement toward the product side. However, in Chapter 10 we discussed a dynamic equilibrium where the rate of evaporation equals the rate of condensation, that is, the reactions are “reversible.”
Many chemical reactions are reversible. The products formed react to give back the original reactants, even as the reactants are forming more products. After some time, both the forward and reverse reactions will be going on at the same rate. When this occurs, the reaction is said to have reached equilibrium. There is no further change in the amount of any reactant or product, though both reactions still go on (forever). Since there are many such reactions that appear to go only partway to completion, their study is of major importance to the chemist.
We will discuss several types of equilibrium in this chapter, along with their associated problems and concepts. You will use the concept of molarity you just learned in Chapter 11 to solve equilibrium problems.
OBJECTIVES
After completing this chapter, you will be able to
· recognize and apply or illustrate: equilibrium, equilibrium constant, equilibrant, equilibrium mixture, homogeneous equilibrium, heterogeneous equilibrium, Le Chatelier's Principle, common ion effect, ion product constant;
· define “reversible reaction” and write an example, and recognize at sight whether a chemical equation is written so as to indicate that the reaction is reversible;
· given a chemical equation, write the equilibrium constant expression;
· given the equilibrium constant expression, write the equation for the corresponding chemical reaction;
· given the equilibrium constant expression, state the units of Keq;
· predict whether changing the temperature of a system in equilibrium will shift the equilibrium to the right or to the left;
· state the effect on a system in chemical equilibrium of removing (or adding) a portion of one reactant (or product) and increasing (or decreasing) the total pressure on a system in equilibrium;
· given the balanced chemical equation for the ionization of a weak acid (or weak base), write the expression for the equilibrium constant;
· given the equilibrium constant expression and all the terms in the equation but one, solve for the unknown;
· given the data needed to calculate concentration terms for the equilibrium calculation, change the data into concentrations;
· given the degree of ionization (percentage ionization or fraction ionized) of a weak acid or weak base, calculate Keq, or given Keq and the necessary concentrations, calculate the degree of ionization;
· write the chemical equation and the ion product constant expression for the ionization of water.
![]() In Chapter 10, you learned about Le Chatelier's Principle in connection with the evaporation and condensation of liquids. Le Chatelier's Principle involved stresses placed upon a dynamic equilibrium. In the case of evaporation and condensation, which are two opposite processes, a dynamic equilibrium occurs when the rate of evaporation and the rate of condensation are equal. Both processes continue, but the rates are equal at equilibrium.
In Chapter 10, you learned about Le Chatelier's Principle in connection with the evaporation and condensation of liquids. Le Chatelier's Principle involved stresses placed upon a dynamic equilibrium. In the case of evaporation and condensation, which are two opposite processes, a dynamic equilibrium occurs when the rate of evaporation and the rate of condensation are equal. Both processes continue, but the rates are equal at equilibrium.
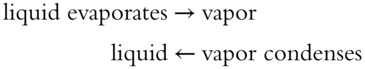
In a dynamic equilibrium, two opposite processes continue to occur, but the rates of the opposite processes must be equal. According to Le Chatelier's Principle, when a system in equilibrium is subjected to a stress, the system will change its conditions, relieving the stress and forming a new equilibrium.
In a covered jar containing a little water, the rates of evaporation and condensation will be equal after a time and equilibrium is achieved. If the covered jar is suddenly pressurized, the rate of condensation will suddenly be increased.
1. What is the stress in this example? __________
2. What will happen to the rate of evaporation to restore dynamic equilibrium?
Answer: (a) pressure (the sudden pressurization of the jar); (b) The rate of evaporation will decrease suddenly. After a time it will again equal the rate of condensation, thus reestablishing equilibrium.
![]() The following two reactions are completely opposite processes.
The following two reactions are completely opposite processes.
![]()
![]()
The reactants of the first reaction are the products in the second reaction and vice versa. Both reactions can be written as one reversible reaction.
![]()
In this reversible reaction, what symbol(s) are used to indicate that the reaction is reversible? __________
Answer: ⇌ (The two arrows pointing in opposite directions indicate that the reaction is reversible.)
![]() Up to this point we have treated all reactions as if they went to completion and continued until one or more of the reactants was consumed. At that point the reaction stopped. However, the reversible reaction in frame 2 proceeds in one direction or the other until an equilibrium is achieved. At equilibrium, all reactants and products are still available in some concentration. Both of the opposite reactions continue at the same rate at equilibrium, but the proportion of reactants and products remains unchanged as long as the equilibrium is maintained.
Up to this point we have treated all reactions as if they went to completion and continued until one or more of the reactants was consumed. At that point the reaction stopped. However, the reversible reaction in frame 2 proceeds in one direction or the other until an equilibrium is achieved. At equilibrium, all reactants and products are still available in some concentration. Both of the opposite reactions continue at the same rate at equilibrium, but the proportion of reactants and products remains unchanged as long as the equilibrium is maintained.
When a reversible reaction goes to equilibrium, do the opposite reactions stop? ______________
Answer: no (Both of the opposite reactions continue at equilibrium but the rates of the two reactions are equal.)
![]() The following reversible reaction:
The following reversible reaction:
![]()
can also be written as:
![]()
By convention, the starting materials of the reaction are written on the left side of the equation. The substances on the left side are also arbitrarily called the reactants, and those on the right side are called the products. The reaction with the arrow pointing to the right (→) is called the forward reaction. The reaction with the arrow pointing to the left (←) is called the reverse reaction.
Write a reversible reaction with the starting material of H2 and I2 (product would be HI). __________
Answer: H2 + I2 ⇌ 2HI
![]() Indicate whether each of the following is a forward or reverse reaction.
Indicate whether each of the following is a forward or reverse reaction.
1. PCl5 → PCl3 + Cl2 __________
2. PCl3 + Cl2 ← PCl5 __________
Answer: (a) forward; (b) reverse
![]() Reversible reactions that go to equilibrium (instead of to completion) end up with an equilibrium mixture of reactants and products after equilibrium is reached. The equilibrium mixture contains various concentrations of each reactant and product. List all the substances found in the equilibrium mixture of the reversible reaction below. ______________________________
Reversible reactions that go to equilibrium (instead of to completion) end up with an equilibrium mixture of reactants and products after equilibrium is reached. The equilibrium mixture contains various concentrations of each reactant and product. List all the substances found in the equilibrium mixture of the reversible reaction below. ______________________________
![]()
Answer: CO2, H2, CO, and H2O (all reactants and products)
![]() When a reversible reaction reaches chemical equilibrium, the concentrations of the components in the equilibrium mixture (the products and reactants) remain constant as long as experimental conditions are not changed. At chemical equilibrium, how does the rate of the forward reaction compare with the rate of the reverse reaction? ______________________
When a reversible reaction reaches chemical equilibrium, the concentrations of the components in the equilibrium mixture (the products and reactants) remain constant as long as experimental conditions are not changed. At chemical equilibrium, how does the rate of the forward reaction compare with the rate of the reverse reaction? ______________________
Answer: The rates are equal.
EQUILIBRIUM CONSTANT
![]() For most reversible reactions going to equilibrium, equilibrium constants have been determined experimentally. An equilibrium constant is the ratio of the concentration of the products divided by the concentration of the reactants at equilibrium and at a specified temperature. The equilibrium constant (product concentrations divided by reactant concentrations) is valid only at a specified temperature after the reaction has gone to (completion, equilibrium) __________
For most reversible reactions going to equilibrium, equilibrium constants have been determined experimentally. An equilibrium constant is the ratio of the concentration of the products divided by the concentration of the reactants at equilibrium and at a specified temperature. The equilibrium constant (product concentrations divided by reactant concentrations) is valid only at a specified temperature after the reaction has gone to (completion, equilibrium) __________
Answer: equilibrium
![]() Below is a reversible reaction and the expression for the equilibrium constant for this reversible reaction.
Below is a reversible reaction and the expression for the equilibrium constant for this reversible reaction.
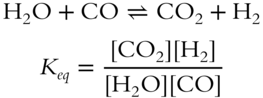
The symbol Keq represents the equilibrium constant and the brackets [] represent the concentration (usually in moles per liter) of each product and reactant. Look at the placement of each reactant and product in the equilibrium constant expression. In the equilibrium constant expression for a reversible reaction, the (products, reactants) ____________ are located in the numerator or upper part of the fraction and the (products, reactants) ________________ are located in the denominator or lower part of the fraction.
Answer: products; reactants
![]() The standard equation, then, for Keq is as follows.
The standard equation, then, for Keq is as follows.
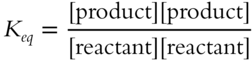
Write the equilibrium constant expression for the following reversible reaction.
![]()
Keq = ___________________
Answer: 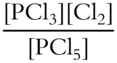
(Since there are two products, they should be placed in the upper part of the fraction. The one reactant belongs in the lower part of the fraction.)
![]() A reversible reaction such as
A reversible reaction such as ![]() has the following equilibrium constant expression.
has the following equilibrium constant expression.
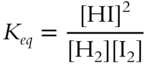
Note that the coefficient in front of HI in the reaction becomes an exponent in the equilibrium constant expression.
Write the equilibrium constant expression for the following reversible reaction.
![]()
Keq = ___________________
Answer: 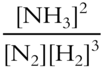
![]() It is easy to prove to yourself that the coefficients in the reaction should be exponents in the expression for an equilibrium constant.
It is easy to prove to yourself that the coefficients in the reaction should be exponents in the expression for an equilibrium constant.
![]()
This reaction can also be written as:
![]()
Write the equilibrium constant expression for this last reaction.
Keq = ___________________
Answer: 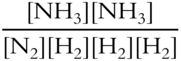
The concentrations of the reactants are multiplied together: [H2][H2][H2] = [H2]3. The concentrations of the products are multiplied together: [NH3][NH3] = [NH3]2. Therefore,
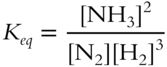
![]() Write the correct equilibrium constant expression for the following reversible reaction.
Write the correct equilibrium constant expression for the following reversible reaction.
![]()
Keq = ___________________
Answer: 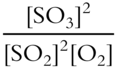
CALCULATING EQUILIBRIUM CONSTANTS
![]() Now let's use this general equation to find some actual equilibrium constants. The equilibrium constant expression for
Now let's use this general equation to find some actual equilibrium constants. The equilibrium constant expression for ![]() is:
is:
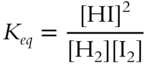
At 458°C, the equilibrium mixture consists of:
![]()
![]()
![]()
Calculate the value of Keq at 458°C (to the nearest tenth).
Keq = ___________________
Answer: 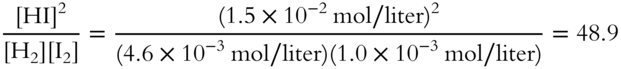
![]() At 458°C, the equilibrium constant (Keq) for the reaction
At 458°C, the equilibrium constant (Keq) for the reaction ![]() is 48.9. If you were to repeat the reaction
is 48.9. If you were to repeat the reaction ![]() using three times as much starting material (H2 and I2) but kept the temperature at 458°C, what would be the value of Keq? __________________
using three times as much starting material (H2 and I2) but kept the temperature at 458°C, what would be the value of Keq? __________________
(Hint: This question is tricky. Remember the meaning of the word constant.)
Answer: 48.9 (Any amount of starting material for this reaction under normal conditions will produce a chemical equilibrium with an equilibrium constant of 48.9. The equilibrium constant for this reaction is a constant at the specified temperature. It does not change.)
![]() After the equilibrium for a reaction is determined experimentally, it is useful for predicting the amount of products at equilibrium or the amount of reactants needed for a different situation involving the same reaction at the specified temperature.
After the equilibrium for a reaction is determined experimentally, it is useful for predicting the amount of products at equilibrium or the amount of reactants needed for a different situation involving the same reaction at the specified temperature.
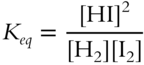
Determine [HI] (the concentration of hydrogen iodide) at equilibrium if:
![]()
![]()
![]()
![]()
Answer: 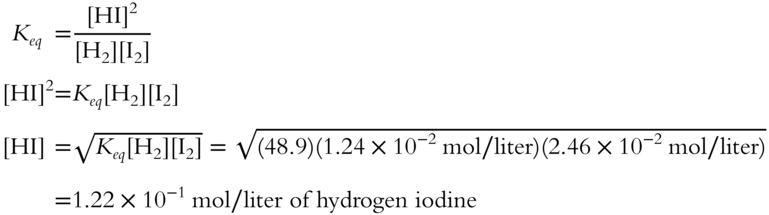
![]() An equilibrium constant expression is valid only if the reaction is balanced. Write the equilibrium constant expression for the following unbalanced reversible reaction. Make sure to balance the reaction equation first. (Refer to Chapter 6, if necessary, for review.)
An equilibrium constant expression is valid only if the reaction is balanced. Write the equilibrium constant expression for the following unbalanced reversible reaction. Make sure to balance the reaction equation first. (Refer to Chapter 6, if necessary, for review.)
![]()
Keq = ___________________
Answer: Balance the reaction equation first (equal numbers of atoms on both sides).
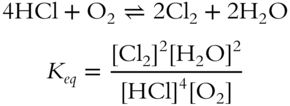
![]() So far, the values of the equilibrium constant (Keq) in all examples have been just numbers because all of the mol/liter concentration terms have canceled out. In determining the value of Keq for the following expression, all mol/liter terms will cancel and Keq will be a unitless number.
So far, the values of the equilibrium constant (Keq) in all examples have been just numbers because all of the mol/liter concentration terms have canceled out. In determining the value of Keq for the following expression, all mol/liter terms will cancel and Keq will be a unitless number.
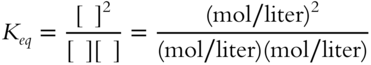
Suppose that for a different reaction, the equilibrium constant expression is as follows.
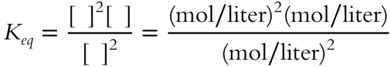
All mol/liter do not cancel in this case. Instead of being just a number, Keq will have a value in terms of _________________.
Answer: mol/liter
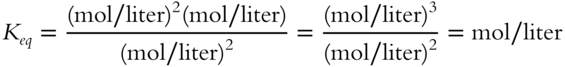
![]() For the following equilibrium constant expression, the value of Keq will not be just a number.
For the following equilibrium constant expression, the value of Keq will not be just a number.
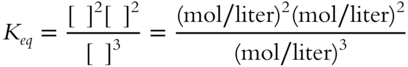
Keq will be expressed in terms of _____________
Answer: mol/liter
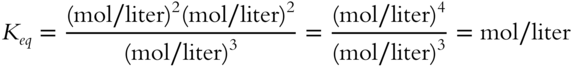
![]() For the following equilibrium constant expression, the value of Keq is not just a number. This example is a bit more difficult than the previous examples since the denominator has the larger exponent.
For the following equilibrium constant expression, the value of Keq is not just a number. This example is a bit more difficult than the previous examples since the denominator has the larger exponent.
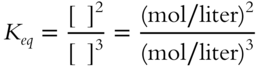
The value of Keq will be in terms of __________
Answer: liters/mol
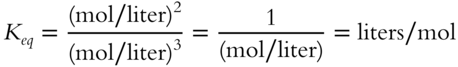
To divide by a fraction, invert the fraction and multiply:
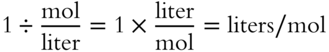
![]() For the following equilibrium constant expression, the value of Keq will not be a pure number.
For the following equilibrium constant expression, the value of Keq will not be a pure number.
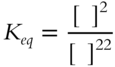
The value of Keq will be in terms of __________.
Answer: ![]()
To divide by a fraction, invert the fraction and multiply:
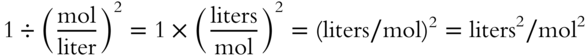
![]() For the following equilibrium constant expression, the value of Keq will not be a pure number.
For the following equilibrium constant expression, the value of Keq will not be a pure number.
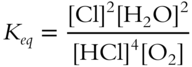
The value of Keq will be in terms of __________.
Answer: 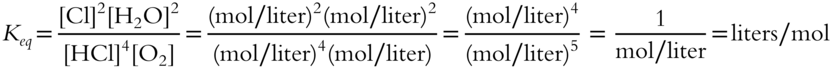
![]() A value of Keq should include proper units such as mol/liter or liters/mol so that further calculations using Keq will have the correct units. Calculate the value of the equilibrium constant for the following reversible reaction.
A value of Keq should include proper units such as mol/liter or liters/mol so that further calculations using Keq will have the correct units. Calculate the value of the equilibrium constant for the following reversible reaction.
![]()
The concentrations of the equilibrants (reactants and products) at equilibrium at 400°C are as follows.
![]()
![]()
![]()
In addition to the numerical value of Keq we must determine the proper units for Keq. After checking the equation for proper balancing, write the expression for the equilibrium constant. After determining the proper expression, you may find it easiest to calculate the numerical value of Keq first and determine the proper units in a separate step.
The value of Keq at 400°C is __________.
Answer: 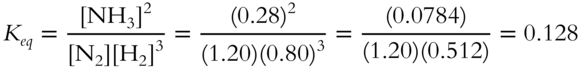
The proper unit can be determined as follows.
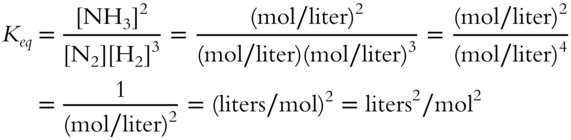
The actual value of Keq is 0.128 liters2/mol2 at 400°C.
TYPES OF EQUILIBRIUM
![]() All of the reversible reactions used as examples have so far included only reactions in which all equilibrants (reactants and products) are of the same state (all gases or all soluble solids). A chemical equilibrium in which all equilibrants are of the same state is called a homogeneous equilibrium. A chemical equilibrium in which the equilibrants are of different states is called a heterogeneous equilibrium.
All of the reversible reactions used as examples have so far included only reactions in which all equilibrants (reactants and products) are of the same state (all gases or all soluble solids). A chemical equilibrium in which all equilibrants are of the same state is called a homogeneous equilibrium. A chemical equilibrium in which the equilibrants are of different states is called a heterogeneous equilibrium.
A chemical equilibrium of a reaction in which a solid and a gas are reactants and a gas is the product is what kind of equilibrium? __________
Answer: heterogeneous
![]() For the following reaction, an abbreviation has been added to indicate the state of each reactant or product.
For the following reaction, an abbreviation has been added to indicate the state of each reactant or product.
![]()
· The letter “g” following a substance indicates that the substance is a gas.
· The letter “l” indicates that a substance is a liquid.
· The letter “s” indicates that a substance is a solid.
· Finally, “aq” indicates that a substance is an ion in aqueous (water) solution.
1. The chemical equilibrium involving the reaction above is what kind of equilibrium? __________
2. An equilibrium involving the following reaction would be what kind of equilibrium? __________
![]()
Answer: (a) homogeneous; (b) heterogeneous
![]() Some heterogeneous equilibrium reactions are special cases when the equilibrium constant expression is written for them.
Some heterogeneous equilibrium reactions are special cases when the equilibrium constant expression is written for them.
![]()
The correct equilibrium constant expression is:
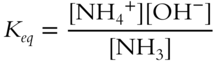
What reactant or product has not been included in the above expression for the equilibrium constant? __________
Answer: H2O
![]() H2O was not included in the equilibrium expression in frame 26 because it is a pure liquid whose concentration does not change significantly. Pure liquids as well as pure solids should not be included in the expression for an equilibrium constant because the concentration of a pure liquid or pure solid does not change if the temperature does not change.
H2O was not included in the equilibrium expression in frame 26 because it is a pure liquid whose concentration does not change significantly. Pure liquids as well as pure solids should not be included in the expression for an equilibrium constant because the concentration of a pure liquid or pure solid does not change if the temperature does not change.
Because the concentrations of pure solids and liquids stay constant, it would be redundant to include them in the equilibrium constant expression. (“Pure” indicates that the compound or element is not mixed with another compound or element. Water is a pure liquid if some other substance is not dissolved or mixed with it.) Gases, however, change concentration with pressure even if the temperature is constant. Only those equilibrants whose concentration is variable should be included in the equilibrium constant expression. Unless otherwise stated, any reactant or product that is a liquid (l) or solid (s) should not be included in the equilibrium constant expression.
Write the equilibrium constant expression for the following reversible reaction.
![]()
(Note that H2O is a vapor that behaves like a gas and not a liquid in this reaction.)
Keq = _____________
Answer: 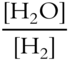
(The CuO and Cu are solids, as indicated by (s) that follows these solids in the equation, and should not be included in the expression.)
![]() Write the equilibrium constant expression for the following reaction.
Write the equilibrium constant expression for the following reaction.
![]()
Keq = _____________
Answer: 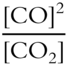
(The solid does not appear in the equilibrium constant expression, gases do.)
![]() Write the equilibrium constant expression for the following reaction.
Write the equilibrium constant expression for the following reaction.
![]()
(Note that H2O is not a liquid in this reaction.)
Keq = _____________
Answer: 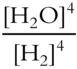
![]() Write the equilibrium constant expression for the following reaction.
Write the equilibrium constant expression for the following reaction.
![]()
Keq = _____________
Answer: [NH3][H2S] (The only reactant is a solid, therefore the denominator is eliminated.)
STRESS IN EQUILIBRIUM
![]() Le Chatelier's Principle is applicable to all chemical equilibria. Such stresses as adding more of a reactant or product to a reaction, or changing the pressure of a reaction involving gases, will have effects that can be predicted through Le Chatelier's Principle. Le Chatelier's Principle states that when a stress is placed on an equilibrium system, the equilibrium will shift so that the stress is (increased, relieved) __________.
Le Chatelier's Principle is applicable to all chemical equilibria. Such stresses as adding more of a reactant or product to a reaction, or changing the pressure of a reaction involving gases, will have effects that can be predicted through Le Chatelier's Principle. Le Chatelier's Principle states that when a stress is placed on an equilibrium system, the equilibrium will shift so that the stress is (increased, relieved) __________.
Answer: relieved
![]() The following reaction can serve as an example of what happens when a stress is placed upon a chemical equilibrium.
The following reaction can serve as an example of what happens when a stress is placed upon a chemical equilibrium.
![]()
First, write the equilibrium constant expression for this reaction.
Keq = _____________
Answer: 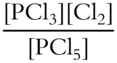
![]() Assume that at a certain temperature, the concentration in mol/liter of each equilibrant in the reaction from frame 32 is as follows.
Assume that at a certain temperature, the concentration in mol/liter of each equilibrant in the reaction from frame 32 is as follows.
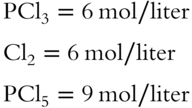
Calculate the value of Keq __________
Answer: 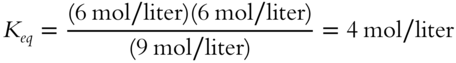
![]() To observe the effects of Le Chatelier's Principle, we now add an additional 6 mol/liter of Cl2 gas to the equilibrium. We suddenly have a total of 12 mol/liter of Cl2 as well as the original amount of PCl3 and PCl5. The system is no longer at equilibrium. Immediately, one of the reaction rates (forward or reverse) increases so as to relieve the stress. Look carefully at the reversible reaction.
To observe the effects of Le Chatelier's Principle, we now add an additional 6 mol/liter of Cl2 gas to the equilibrium. We suddenly have a total of 12 mol/liter of Cl2 as well as the original amount of PCl3 and PCl5. The system is no longer at equilibrium. Immediately, one of the reaction rates (forward or reverse) increases so as to relieve the stress. Look carefully at the reversible reaction.
![]()
Since the concentration of Cl2 has just doubled, the reaction will try to relieve that stress. As a response to all that extra Cl2, which of the following would you expect to happen?
1. The forward reaction rate increases (producing even more Cl2 and PCl3 while reducing the PCl5).
2. The reverse reaction increases (reducing the supply of Cl2 and PCl3 while increasing the PCl5).
Answer: (b) (This is logical since there is a surplus of Cl2. The reverse reaction rate increases until equilibrium is again restored.)
![]() After relieving the stress, the reaction from frames 32—34 will again be at equilibrium, although the concentration of each equilibrant has changed.
After relieving the stress, the reaction from frames 32—34 will again be at equilibrium, although the concentration of each equilibrant has changed.
![]()
1. Original Keq:
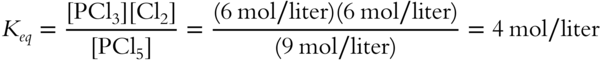
2. Doubling the Cl2 concentration, the system is no longer at equilibrium:
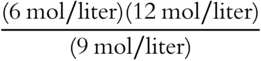
After doubling the Cl2 concentration, the system returns to equilibrium.
The concentration of each equilibrant at the new equilibrium has been calculated for you using a mathematical procedure called the quadratic equation. You will not be required to use quadratic equations in this text.
3. New Keq:
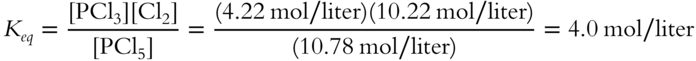
Note that after equilibrium is restored, the value for Keq remains the same. Since the temperature has remained the same, should you have expected the value for Keq to change? ____________
Answer: no (If the temperature does not change, Keq will not change because it is a constant for the reaction at equilibrium.)
![]() Compare the new equilibrium concentrations to those of the original equilibrium in frame 35. Besides the doubled Cl2 concentration (which was the stress applied), which equilibrant
Compare the new equilibrium concentrations to those of the original equilibrium in frame 35. Besides the doubled Cl2 concentration (which was the stress applied), which equilibrant
1. increased in concentration? __________
2. decreased in concentration? __________
Answer: (a) The concentration of PCl5 increased from 9 mol/liter to 10.78 mol/liter; (b) The concentration of PCl3 decreased from 6 mol/liter to 4.22 mol/liter.
![]() Adding more of one of the products (Cl2) in the above reaction resulted in a shift of the equilibrium that favored the reverse reaction, as predicted by Le Chatelier's Principle.
Adding more of one of the products (Cl2) in the above reaction resulted in a shift of the equilibrium that favored the reverse reaction, as predicted by Le Chatelier's Principle.
After equilibrium is again restored, the concentration of the other product (PCl3) is (decreased, increased) ___________. The concentration of the reactant (PCl5) is (decreased, increased) __________.
Answer: decreased; increased
![]() As a general rule (because of Le Chatelier's Principle), increasing the concentration of one of the products of a reaction favors the reverse reaction and results in a decrease in the concentration of any other product and an increase in the concentration of the reactants. This reaction represents an ordinary reversible reaction at equilibrium.
As a general rule (because of Le Chatelier's Principle), increasing the concentration of one of the products of a reaction favors the reverse reaction and results in a decrease in the concentration of any other product and an increase in the concentration of the reactants. This reaction represents an ordinary reversible reaction at equilibrium.
![]()
If the concentration of product D is suddenly increased, label each of the following as increased or decreased.
1. the concentration of A __________
2. the concentration of B __________
3. the concentration of C __________
Answer: (a) increased; (b) increased; (c) decreased (The equilibrium is shifted to favor the reactants.)
![]() The reversible reaction A + B ⇌ C + D can also be written C + D ⇌ A + B. Suppose the concentration of A were increased suddenly. After equilibrium is again restored, which of the following increases and which decreases?
The reversible reaction A + B ⇌ C + D can also be written C + D ⇌ A + B. Suppose the concentration of A were increased suddenly. After equilibrium is again restored, which of the following increases and which decreases?
1. the concentration of B __________
2. the concentration of C __________
3. the concentration of D __________
Answer: (a) decreases; (b) increases; (c) increases
![]() According to Le Chatelier's Principle:
According to Le Chatelier's Principle:
· If the concentration of one reactant is suddenly increased, the equilibrium will shift so that all products are increased and the other reactant will be decreased.
· If the concentration of one product is suddenly increased, the equilibrium will shift so that all reactants are increased and the other product is decreased.
The brackets [ ] indicate concentration (in mol/liter).
![]()
If [C] is increased, then [A] is ______________, [B] is _______________, and [D] is _______________.
Answer: increased; increased; decreased
![]() Decreasing a reactant or product can also be a stress.
Decreasing a reactant or product can also be a stress.
![]()
If C were to be suddenly decreased by removing some from the reaction system, the forward reaction would be favored and more product would be produced until equilibrium was again established. To produce more product would require a decreased concentration of reactants. A decrease in [C] causes the exact opposite of an increase in [C].
1. If [A] is increased (by adding some A to the reaction mixture), then [B] is ________________, [C] is _______________, and [D] is ______________.
2. If [A] is decreased (by removing some A from the reaction mixture), then [B] is ________________, [C] is _______________, and [D] is ______________.
Answer: (a) decreased; increased; increased (The forward reaction is favored.); (b) increased; decreased; decreased (The reverse reaction is favored.)
![]() Whenever a quantity of a reactant or product is added to or removed from a chemical equilibrium system, the equilibrium responds according to Le Chatelier's Principle. The stress in this case is the addition or removal of some quantity of reactant or product. A chemical equilibrium will also respond to a change in temperature.
Whenever a quantity of a reactant or product is added to or removed from a chemical equilibrium system, the equilibrium responds according to Le Chatelier's Principle. The stress in this case is the addition or removal of some quantity of reactant or product. A chemical equilibrium will also respond to a change in temperature.
A chemical reaction may either require heat to continue the reaction or give off heat during the reaction. A chemical reaction requiring heat (energy) to proceed is called endothermic. A chemical reaction giving off heat (energy) during a reaction is exothermic.
The forward reaction CaCO3(s) ⇌ CaO(s) + CO2(g) requires heat energy. It is an (exothermic, endothermic) __________ reaction.
Answer: endothermic
![]() The forward reaction of N2(g) + 3H2(g) ⇌ 2NH3(g) is exothermic. It (gives off, requires) __________ heat energy.
The forward reaction of N2(g) + 3H2(g) ⇌ 2NH3(g) is exothermic. It (gives off, requires) __________ heat energy.
Answer: gives off
![]() A forward exothermic reaction can be written to show heat as a product.
A forward exothermic reaction can be written to show heat as a product.
![]()
A forward endothermic reaction can be written to show heat as a requirement on the reactant side. The following forward reaction is endothermic. Which equation is correctly written?
· _____ (a) heat + CaCO3(s) ⇌ CaO(s) + CO2(g)
· _____ (b) CaCO3(s) ⇌ Cao + CO2(g) + heat
Answer: (a)
![]() The equilibrium constant for an endothermic reaction increases with rising temperature. The equilibrium constant for an exothermic reaction decreases with rising temperature. This means that for an endothermic reaction, increasing the temperature will increase the concentration of products (at the expense of reactants). For an exothermic reaction, the opposite holds true.
The equilibrium constant for an endothermic reaction increases with rising temperature. The equilibrium constant for an exothermic reaction decreases with rising temperature. This means that for an endothermic reaction, increasing the temperature will increase the concentration of products (at the expense of reactants). For an exothermic reaction, the opposite holds true.
A simple way to remember these effects is to treat the heat energy as a reactant or product and apply what you have learned from Le Chatelier's Principle.
![]()
Treating heat as a required item on the reactant side, apply Le Chatelier's Principle. If the temperature is raised (heat is increased), indicate whether the concentrations of the following reactant and products increase or decrease.
1. [CaCO3] __________
2. [CaO] __________
3. [CO2] __________
4. Is the reaction endothermic or exothermic? __________
Answer: (a) decreases; (b) increases; (c) increases; (d) endothermic (Increasing the required heat energy favors the forward reaction.)
![]() Indicate whether increasing the temperature for the reaction expressed below would increase or decrease the concentrations of the reactants and product.
Indicate whether increasing the temperature for the reaction expressed below would increase or decrease the concentrations of the reactants and product.
![]()
1. [NH3] __________
2. [N2] __________
3. [H2] __________
4. Is this reaction endothermic or exothermic? __________
Answer: (a) decreases; (b) increases; (c) increases; (d) exothermic (Increasing one of the products would favor the reverse reaction. Since heat energy is being treated as a product, increasing it would have the same effect.)
![]() A decrease in temperature (some of the heat is removed) has an effect opposite to that of an increase.
A decrease in temperature (some of the heat is removed) has an effect opposite to that of an increase.
![]()
If the temperature is decreased, indicate whether the concentrations of the reactants and product would increase or decrease.
1. [NH3] __________
2. [N2] __________
3. [H2] __________
Answer: (a) increases; (b) decreases; (c) decreases (Decreasing one of the products favors the forward reaction. Since heat is being treated as a product, decreasing it has the same effect.)
![]() The effects of increasing or decreasing the pressure surrounding a chemical equilibrium involving gases can also be predicted on the basis of Le Chatelier's Principle. Increasing the pressure favors the side of the reaction (reactant side or product side) containing the fewest moles of gas.
The effects of increasing or decreasing the pressure surrounding a chemical equilibrium involving gases can also be predicted on the basis of Le Chatelier's Principle. Increasing the pressure favors the side of the reaction (reactant side or product side) containing the fewest moles of gas.
For the following reaction, N2(g) + 3H2(g) ⇌ 2NH3(g), the reactant side has 4 mol of gas and the product side has 2 mol of gas. If the pressure is increased, which side will be favored and end up with an increase in concentration? ______________
Answer: the product side (Increased pressure favors the reaction side with the fewest moles of gas.)
![]() In this reaction, only the CO2 product is a gas. The other equilibrants are solids that cannot be compressed.
In this reaction, only the CO2 product is a gas. The other equilibrants are solids that cannot be compressed.
![]()
An increase in pressure causes the reaction equilibrium to shift in favor of the side of the reaction with the least moles of gas. In this reaction, the reactant side has no moles of gas while the product side has 1 mol of gas. An increase in pressure would favor which side of the reaction? __________
Answer: the reactant side (Zero moles of gas is less gas than 1 mol of gas.)
![]() Now look at this reaction.
Now look at this reaction.
![]()
1. The reactant side of this reaction has how many moles of gas? __________
2. The product side has how many moles of gas? __________
3. Which side of the reaction would be favored by an increase in pressure?
4. Which side of the reaction would be favored by a decrease in pressure? (Remember, decreasing the pressure has the opposite effect of increasing the pressure.) __________
Answer: (a) 1 mol; (b) 2 mol (PCl3 and Cl2 each represent 1 mol of gas, which adds up to 2 mol on the product side.); (c) reactant side; (d) product side
![]() Here's a tricky question. Remember, increasing the pressure favors the side of the reaction with the least moles of gas, provided there is a side with the least moles of gas. For the following reversible reaction, increasing the pressure will favor which side of the reaction? __________
Here's a tricky question. Remember, increasing the pressure favors the side of the reaction with the least moles of gas, provided there is a side with the least moles of gas. For the following reversible reaction, increasing the pressure will favor which side of the reaction? __________
![]()
Answer: Neither side is favored because there are 2 mol of gas on each side of the reaction. (Increasing the pressure has no effect if both sides of a reaction have an equal number of moles of gas.)
![]() The reversible reaction below is presently at equilibrium. You desire to shift the equilibrium to favor the product side in order to increase the concentration of CO2 as much as possible.
The reversible reaction below is presently at equilibrium. You desire to shift the equilibrium to favor the product side in order to increase the concentration of CO2 as much as possible.
![]()
To produce an equilibrium with a large concentration of CO2, indicate whether you would increase or decrease the following conditions.
1. pressure __________
2. temperature __________
3. concentration of O2(g) __________
Answer: (a) increase (This favors the product side since it has fewer moles of gas.); (b) decrease (Treating heat as a product, this removes some heat favoring the production of more product.); (c) increase (Increasing the concentration of a reactant results in an increase in products as well as a decrease in other reactants.)
We have discussed the concept of equilibrium and how it applies to those chemical reactions that do not go to completion. You have learned what happens to a reaction at equilibrium when a “stress” is applied to the reaction. Most of the equilibria discussed involved reactants and products that were all in the gaseous state or equilibria between gases and pure solids and liquids.
Chemists have learned that many reactions that occur between ions in aqueous solutions also do not go to completion and reach a state of equilibrium. Ionic equilibria are also affected by stresses according to Le Chatelier's Principle.
We now discuss several special cases of ionic equilibria and how they are used to determine the degree of dissociation of weak electrolytes and the concentration of ions in aqueous solutions. We also discuss how slightly soluble compounds behave and how their solubility is determined in aqueous solutions and in the presence of other ions.
IONIC EQUILIBRIA
![]() You have used Le Chatelier's Principle and the equilibrium constant for reversible reactions. Equilibria also exist for such things as salts and their ions in solution, and acids and bases and their dissociated ions. Other substances, even though they may dissociate only very slightly into ions, are at equilibrium with those ions. An ionic equilibrium constant expression is written just like those of the reversible reactions previously encountered. Write the ionic equilibrium constant for the following reaction.
You have used Le Chatelier's Principle and the equilibrium constant for reversible reactions. Equilibria also exist for such things as salts and their ions in solution, and acids and bases and their dissociated ions. Other substances, even though they may dissociate only very slightly into ions, are at equilibrium with those ions. An ionic equilibrium constant expression is written just like those of the reversible reactions previously encountered. Write the ionic equilibrium constant for the following reaction.
![]()
Keq = ____________
Answer: 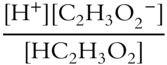
![]() Instead of Keq, the equilibrium constant may be Ka if it represents the dissociation of an acid, Kb if it represents the dissociation of a base, Ki for a general ionization constant, or Ksp for a solubility product constant. We will discuss Ksp now and these other constants later. The equilibrium constant expression is the same for any of these as for the reactions dealt with previously. Write the equilibrium constant expression for the solubility product of the very slightly soluble salt AgBr.
Instead of Keq, the equilibrium constant may be Ka if it represents the dissociation of an acid, Kb if it represents the dissociation of a base, Ki for a general ionization constant, or Ksp for a solubility product constant. We will discuss Ksp now and these other constants later. The equilibrium constant expression is the same for any of these as for the reactions dealt with previously. Write the equilibrium constant expression for the solubility product of the very slightly soluble salt AgBr.
![]()
Ksp = ____________
Answer: [Ag+][Br−] (The solid is not included since its concentration is constant at constant temperature.)
![]() The abbreviation Ksp stands for the solubility product constant. It represents the solubility of a salt in a saturated solution and is called a product because the negative and positive ion concentrations are multiplied together to determine the value of the constant. Write the solubility product expression for the salt CaF2.
The abbreviation Ksp stands for the solubility product constant. It represents the solubility of a salt in a saturated solution and is called a product because the negative and positive ion concentrations are multiplied together to determine the value of the constant. Write the solubility product expression for the salt CaF2.
![]()
Ksp = ____________
Answer: [Ca2+][F−]2 (Don't forget the exponent.)
![]() The solubility product equation represents the dynamic equilibrium between a solid and its dissociated ions in a saturated solution.
The solubility product equation represents the dynamic equilibrium between a solid and its dissociated ions in a saturated solution.
![]()
Write the solubility product expression for the saturated aqueous solution of Bi2S3.
Ksp = ____________
Answer: [Bi3+]2[S2−]3
![]() Write the Ksp expression for the saturated aqueous solution of AgCl. (AgCl is only slightly soluble.)
Write the Ksp expression for the saturated aqueous solution of AgCl. (AgCl is only slightly soluble.)
![]()
Ksp = ____________
Answer: [Ag+][Cl−]
![]() In a saturated solution of slightly soluble AgCl in water, an equilibrium exists between the AgCl solid and its dissociated aqueous ions.
In a saturated solution of slightly soluble AgCl in water, an equilibrium exists between the AgCl solid and its dissociated aqueous ions.
![]()
Suppose that extra Cl− ion is added to this equilibrium. From Le Chatelier's Principle, you know that the equilibrium would favor the left side of the equation. This situation is like the general reversible reaction A + B ⇌ C + D. Adding more Cl− ion to the equilibrium is like adding more of product D to the reaction. Adding more of product D causes an increase in the concentration of the reactants and a decrease in the concentration of product C. Indicate whether adding more Cl− ion to the equilibrium causes an increase or decrease in the following:
1. the formation of AgCl(s) __________
2. the concentration of Ag+ __________
Answer: (a) increase; (b) decrease
![]() The effect of adding more Cl− to the saturated solution of AgCl can also be shown by using the solubility product constant. The solubility of AgCl is 1.2 × 10−5 mol/liter.
The effect of adding more Cl− to the saturated solution of AgCl can also be shown by using the solubility product constant. The solubility of AgCl is 1.2 × 10−5 mol/liter.
![]()
![]()
Assume the Ksp to equal 1.44 × 10−10 mol2/liter2. If the Cl− concentration were to be increased, what must happen to the Ag+ concentration? (Remember that Ksp is a constant and does not change.) ______________
Answer: The Ag+ concentration must decrease. (When the Ag+ concentration and Cl— concentration are multiplied together, they must equal the Ksp constant. When the concentration of one of the ions is increased, the other must decrease to keep the Ksp constant.)
![]() The extra Cl− ion that is added to the saturated solution of AgCl of frames 58 and 59 might have come from NaCl or some other strong electrolyte having a Cl− ion. This is an example of the common ion effect. The slightly soluble AgCl and the strong electrolyte NaCl both have the Cl− ion in common. Adding some NaCl to a saturated solution of AgCl forces the equilibrium to shift so that some of the Ag+(aq) and the extra Cl−(aq) come out of the solution and precipitate. In this example of the common ion effect, which is the “common” ion? __________
The extra Cl− ion that is added to the saturated solution of AgCl of frames 58 and 59 might have come from NaCl or some other strong electrolyte having a Cl− ion. This is an example of the common ion effect. The slightly soluble AgCl and the strong electrolyte NaCl both have the Cl− ion in common. Adding some NaCl to a saturated solution of AgCl forces the equilibrium to shift so that some of the Ag+(aq) and the extra Cl−(aq) come out of the solution and precipitate. In this example of the common ion effect, which is the “common” ion? __________
Answer: Cl− (from both NaCl and AgCI)
![]() If some of the strong electrolyte AgNO3 is added to a saturated solution of AgCl, the common ion effect can also be observed.
If some of the strong electrolyte AgNO3 is added to a saturated solution of AgCl, the common ion effect can also be observed.
![]()
In this example of the common ion effect, which would be the “common” ion? ___________
Answer: Ag+ (from both AgCI and AgNO3)
![]() If you added some strong electrolyte such as NaNO3 to a saturated solution of AgCl, would you expect to observe the common ion effect? __________
If you added some strong electrolyte such as NaNO3 to a saturated solution of AgCl, would you expect to observe the common ion effect? __________
Answer: no (There are not two ions alike in AgCI and NaNO3.)
![]() The common ion effect is observed when a strong electrolyte is added to a saturated solution of a very slightly soluble salt, weak acid, or weak base, and one ion of the strong electrolyte is the same as one of the ions in the original solution.
The common ion effect is observed when a strong electrolyte is added to a saturated solution of a very slightly soluble salt, weak acid, or weak base, and one ion of the strong electrolyte is the same as one of the ions in the original solution.
You may have wondered why AgCl is used as an example since we have previously assumed that all of AgCl precipitated out of solution. In actuality, all salts are soluble to some degree. The solubility of some salts such as AgCl is so minimal that very little error is introduced by treating it elsewhere as not soluble at all.
Is there a salt that is absolutely not soluble even to a small degree? ________
Answer: no (All salts are soluble to some slight degree. For practical purposes in some experiments, we can often ignore the dissolved portion of a very slightly soluble salt. For Ksp calculations and the common ion effect, we cannot ignore it.)
![]() The value of the solubility product constant (Ksp) can easily be determined if the solubility of a salt is known.
The value of the solubility product constant (Ksp) can easily be determined if the solubility of a salt is known.
![]()
Lead sulfide (PbS) is a slightly soluble salt. Only 1 × 10−14 mol of the salt will dissolve in a liter of water at 20 °C. In pure water, at 20 °C, this means that the concentration of Pb2+ ion will be 1 × 10−14 mol/liter and the concentration of S2− ion will also be 1 × 10−14 mol/liter. Calculate Ksp
Ksp = [Pb2+][S2−] = ____________
Answer: (1 × 10−14 mol/liter)(1 × 10−14 mol/liter) = 1 × 10−28 mol2/liter2
![]() Up to 7 × 10−5 mol of calcium carbonate will dissolve in a liter of pure water at 20°C.
Up to 7 × 10−5 mol of calcium carbonate will dissolve in a liter of pure water at 20°C.
![]()
Calculate the Ksp of CaCO3 at 20°C.
Ksp = _________________
Answer: [Ca2+][CO32−] = (7 × 10−5 mol/liter)(7 × 10−5 mol/liter) = 4.9 × 10−9 mol2/liter2
![]() At a certain temperature, up to 2 × 10−4 mol of CaF2 will dissolve in a liter of pure water. Calculate the Ksp of CaF2 at that temperature.
At a certain temperature, up to 2 × 10−4 mol of CaF2 will dissolve in a liter of pure water. Calculate the Ksp of CaF2 at that temperature.
![]()
(Note: The fluoride ion has a coefficient of 2 in front of it. This means that two fluoride ions are produced for every calcium ion when CaF2 dissociates. It also means that the fluoride ion concentration must be squared in the Ksp expression.)
Ksp = _________________
Answer: [Ca2+] = 2 × 10−4 mol/liter
Two fluoride ions are formed for every calcium ion.
![]()
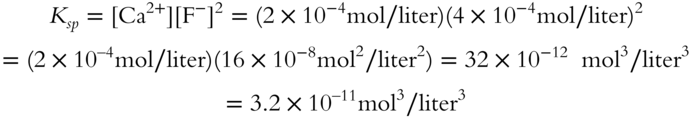
![]() All of the Ksp solubilities calculated in the examples assume that the salts are dissolved in pure water (with no common ions present). Once the Ksp is determined, however, we can use the constant to determine the concentration of one ion of the salt if the concentration of the other ion is known.
All of the Ksp solubilities calculated in the examples assume that the salts are dissolved in pure water (with no common ions present). Once the Ksp is determined, however, we can use the constant to determine the concentration of one ion of the salt if the concentration of the other ion is known.
From a table of Ksp values, the value of the Ksp for iron sulfide (FeS) is 1 × 10−22 mol2/liter2 at a certain temperature.
![]()
First, write the expression for the Ksp of FeS.
Ksp = _____________
Answer: [Fe2+][S2−] = 1 × 10−22 mol2/liter2
![]() A liter of water solution already contains some small quantity of Fe2+ in it. The water is at the proper temperature. By experiment, it is determined that a maximum of 1 × 10−3 mol of S2− can be mixed in this liter of water solution before the FeS precipitate is formed. Since the value for Ksp is known and the concentration of S2− is 1 × 10−3 mol/liter, we can determine the concentration of Fe2+ in the water sample by modifying the Ksp expression.
A liter of water solution already contains some small quantity of Fe2+ in it. The water is at the proper temperature. By experiment, it is determined that a maximum of 1 × 10−3 mol of S2− can be mixed in this liter of water solution before the FeS precipitate is formed. Since the value for Ksp is known and the concentration of S2− is 1 × 10−3 mol/liter, we can determine the concentration of Fe2+ in the water sample by modifying the Ksp expression.
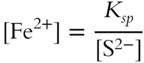
Calculate the concentration of Fe2+. _________________
Answer: 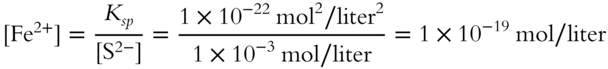
![]() The Ksp for BaCO3 is 5 × 10−9 mol2/liter2 at 20°C.
The Ksp for BaCO3 is 5 × 10−9 mol2/liter2 at 20°C.
![]()
An aqueous solution contains a Ba2+ concentration of 2 × 10−4 mol/liter. What is the maximum possible ![]() concentration in that solution just before precipitation of BaCO3 occurs? The solution temperature is kept at 20°C.
concentration in that solution just before precipitation of BaCO3 occurs? The solution temperature is kept at 20°C.
[![]() ] = __________
] = __________
Answer: 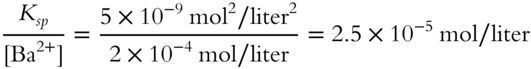
![]() Determine the maximum possible F− ion concentration in an aqueous solution containing 1 × 10−3 mol/liter of Ca2+ ion. The Ksp for CaF2 is equal to 4 × 10−11 mol3/liter3 at the same temperature as the solution. (This one is more difficult. Remember to square the F− in the Ksp expression.)
Determine the maximum possible F− ion concentration in an aqueous solution containing 1 × 10−3 mol/liter of Ca2+ ion. The Ksp for CaF2 is equal to 4 × 10−11 mol3/liter3 at the same temperature as the solution. (This one is more difficult. Remember to square the F− in the Ksp expression.)
![]()
[F−] = __________
Answer:
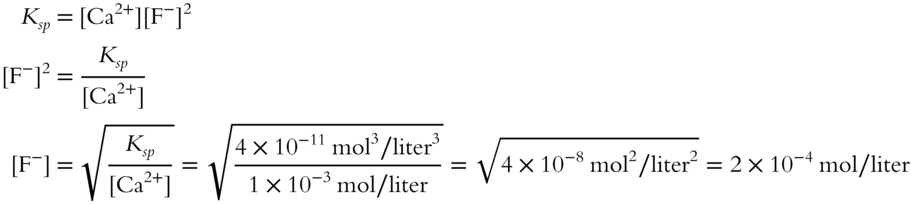
![]() Water is usually considered to be completely a nonelectrolyte. Even water, however, dissociates to a very small degree. This is called self-ionization and will be covered in more detail in the next chapter. The dissociation of water is represented by the following equation.
Water is usually considered to be completely a nonelectrolyte. Even water, however, dissociates to a very small degree. This is called self-ionization and will be covered in more detail in the next chapter. The dissociation of water is represented by the following equation.
![]()
Write the ion product constant expression (Kw) for the dissociation of water.
Kw = _____________
Answer: [H+][OH−] (Liquid water is left out since its concentration essentially does not change.)
![]() The actual value of the ion product constant for water (Kw) is 1 × 10−14 mol2/liter2 (at 25°C). You should memorize the value of Kw since it is very commonly used in aqueous acid and base calculations. In water with no impurities (at 25°C) the concentration of H+ is 1 × 10−7 mol/liter and the concentration of OH− is also 1 × 10−7 mol/liter. Calculate the value of Kw with these concentrations.
The actual value of the ion product constant for water (Kw) is 1 × 10−14 mol2/liter2 (at 25°C). You should memorize the value of Kw since it is very commonly used in aqueous acid and base calculations. In water with no impurities (at 25°C) the concentration of H+ is 1 × 10−7 mol/liter and the concentration of OH− is also 1 × 10−7 mol/liter. Calculate the value of Kw with these concentrations.
Kw = (1 × 10−7 mol/liter)(1 × 10−7 mol/liter) = __________
Answer: 1 × 10−14 mol2/liter2
![]() The most common definition of an acid or base depends upon the concentration of the hydrogen ion in an aqueous solution. In pure water, the concentrations of H+ and OH− are equal. It is neutral (neither acidic nor basic). If the H+ ion concentration is greater than 1 × 10−7 mol/liter, the solution is acidic. If the H+ ion concentration is less than 1 × 10−7 mol/liter, the solution is basic.
The most common definition of an acid or base depends upon the concentration of the hydrogen ion in an aqueous solution. In pure water, the concentrations of H+ and OH− are equal. It is neutral (neither acidic nor basic). If the H+ ion concentration is greater than 1 × 10−7 mol/liter, the solution is acidic. If the H+ ion concentration is less than 1 × 10−7 mol/liter, the solution is basic.
A water solution is found with [H+] = 1 × 10−5 mol/liter. The solution is (acidic, basic) __________.
Answer: acidic (A concentration of 1 × 10−5 is greater than 1 × 10−7.)
![]() If the [H+] is 1 × 10−5 mol/liter for a particular solution, the [OH−] can be calculated.
If the [H+] is 1 × 10−5 mol/liter for a particular solution, the [OH−] can be calculated.
![]()
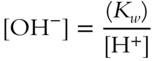
Calculate the concentration of OH− for this solution.
[OH−] = _____________
Answer: 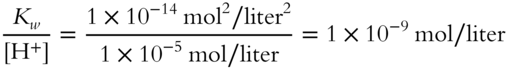
![]() In Chapter 13, acids and bases will be covered in greater detail. For now, an acid is considered to be a substance that increases the H+ concentration of neutral water. A base is considered to be a substance that decreases the H+ concentration of neutral water. The strength of an acid or base depends only upon the extent of dissociation when in water (aqueous) solution. The same definition is already familiar to you as a strong electrolyte or a weak electrolyte. A strong acid (or base) is a strong electrolyte that dissociates completely into its ions in aqueous solution. A weak acid (or base) is a weak electrolyte that dissociates only partially in aqueous solution.
In Chapter 13, acids and bases will be covered in greater detail. For now, an acid is considered to be a substance that increases the H+ concentration of neutral water. A base is considered to be a substance that decreases the H+ concentration of neutral water. The strength of an acid or base depends only upon the extent of dissociation when in water (aqueous) solution. The same definition is already familiar to you as a strong electrolyte or a weak electrolyte. A strong acid (or base) is a strong electrolyte that dissociates completely into its ions in aqueous solution. A weak acid (or base) is a weak electrolyte that dissociates only partially in aqueous solution.
HCl is an acid that is a strong electrolyte.
![]()
Remember that M indicates molarity (moles/liter). If a 0.1 M solution of HCl dissociates, it produces a ________M solution of H+ and a ________M solution of Cl−.
Answer: 0.1; 0.1
![]() HCl is such a strong electrolyte that in an aqueous solution, there are only H+ and Cl− ions. The equation for the dissociation of HCl is:
HCl is such a strong electrolyte that in an aqueous solution, there are only H+ and Cl− ions. The equation for the dissociation of HCl is:
![]()
In aqueous solution, there is no more HCl; only H+ and Cl− ions can be found. The equation for the dissociation of HCl goes to completion. Only the products are left. The reactant (HCl) is completely used up to form products. An equation that goes to completion must be distinguished from an equilibrium equation. In an equilibrium equation, both the products and the reactants are continually present and the forward and reverse reactions continue at equal rates.
An aqueous solution of 0.001 M HCl is actually dissociated into a solution of 0.001 M H+ and 0.001 M Cl−.
Ignoring the chloride ion, a solution of 0.001 M H+ is the same as [H+] = 1 × 10−3 mol/liter by definition.
1. A solution of 0.01 M HCl results in [H+] = __________
2. After being dissociated into ions in an aqueous solution, would you expect to find any undissociated HCl in the 0.01 M solution? __________
Answer: (a) 0.01 M, or 1 × 10−2 M, or 1 × 10−2 mol/liter (all are correct answers); (b) No, the HCI dissociates completely into ions.
![]() Not all acids are strong electrolytes. Some are weak electrolytes that only dissociate partly into ions. Part of the weak electrolyte remains undissociated. This part establishes equilibrium with the dissociated ions. The equilibrium constant for a weak acid is abbreviated Ka.
Not all acids are strong electrolytes. Some are weak electrolytes that only dissociate partly into ions. Part of the weak electrolyte remains undissociated. This part establishes equilibrium with the dissociated ions. The equilibrium constant for a weak acid is abbreviated Ka.
Acetic acid is an example of a weak acid. What is the acetic acid equilibrium constant expression?
![]()
Ka = _____________________
Answer: 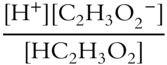
![]() At 0.02 mol/liter concentration, acetic acid is 3% dissociated. Therefore, the hydrogen ion concentration of the solution equals 3% multiplied by 0.02 mol/liter. (The 3% becomes 0.03 in decimal form.)
At 0.02 mol/liter concentration, acetic acid is 3% dissociated. Therefore, the hydrogen ion concentration of the solution equals 3% multiplied by 0.02 mol/liter. (The 3% becomes 0.03 in decimal form.)
![]()
The acetate ion concentration [![]() ] equals the hydrogen ion concentration [H+] since one acetate ion is formed for every hydrogen ion. What is the concentration of the hydrogen ion?
] equals the hydrogen ion concentration [H+] since one acetate ion is formed for every hydrogen ion. What is the concentration of the hydrogen ion?
[H+] = (0.03)(0.02 mol/liter) = __________
Answer: (The solution is made up of 0.02 mol/liter HC2H3O2 acid. If the acid were to dissociate completely (100%), the value of [H+] would also be 0.02 mol/liter. However, the acid only dissociates 3%. Therefore, the hydrogen ion concentration will be 3% of 0.02 mol/liter.)
![]()
![]() Acetic acid is 3% dissociated in a 0.02 mol/liter solution. Therefore,
Acetic acid is 3% dissociated in a 0.02 mol/liter solution. Therefore,
![]()
![]()
The remaining 97% is molecular acetic acid. The 97% becomes 0.97 in decimal form. The concentration of the remaining molecular acetic acid can be found by multiplying 0.97 times 0.02 mol/liter.
[HC2H3O2] = (0.97)(0.02 mol/liter) = __________
Answer: 0.0194 mol/liter or 1.94 × 10−2 mol/liter
![]() Calculate the ionization constant (Ka) for acetic acid. (Round to the nearest hundredth.)
Calculate the ionization constant (Ka) for acetic acid. (Round to the nearest hundredth.)
![]()
![]()
![]()

Answer: 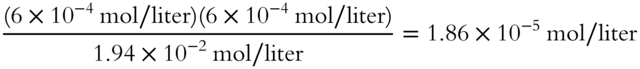
![]() A solution of 0.050 M hydrofluoric acid (HF) is found to be 10% dissociated (or ionized). If the acid were to dissociate completely (100%), the value of [H+] would also be 0.050 M. However, the acid only dissociates 10%. Therefore, the hydrogen ion concentration will be 10% of 0.050 M. The 10% converts to a decimal of 0.10 in the calculation. The portion of HF that dissociates produces one H+ ion for each F− ion.
A solution of 0.050 M hydrofluoric acid (HF) is found to be 10% dissociated (or ionized). If the acid were to dissociate completely (100%), the value of [H+] would also be 0.050 M. However, the acid only dissociates 10%. Therefore, the hydrogen ion concentration will be 10% of 0.050 M. The 10% converts to a decimal of 0.10 in the calculation. The portion of HF that dissociates produces one H+ ion for each F− ion.
![]()
Determine the concentrations (expressed as M, molarity) of H+ and F− in the solution.
[H+] = _____________
[F−] = ____________
Answer: (0.10)(0.050 M) = 0.005 M or 5 × 10−3 M(0.10)(0.050 M) = 0.005 M or 5 × 10−3 M
![]() The HF acid solution is 0.050 M. If 90% of the acid remains in molecular form, calculate the concentration of the acid remaining in molecular form. (The 90% becomes 0.90 in decimal form.)
The HF acid solution is 0.050 M. If 90% of the acid remains in molecular form, calculate the concentration of the acid remaining in molecular form. (The 90% becomes 0.90 in decimal form.)
[HF] = __________
Answer: (0.90)(0.050 M) = 4.5 × 10−2 M
![]() Write the ionization constant expression (Ka) for hydrofluoric acid and calculate the value of Ka using the various concentrations just calculated in the previous two frames. (Round to the nearest tenth.)
Write the ionization constant expression (Ka) for hydrofluoric acid and calculate the value of Ka using the various concentrations just calculated in the previous two frames. (Round to the nearest tenth.)
![]()
Ka = ______________
Answer: 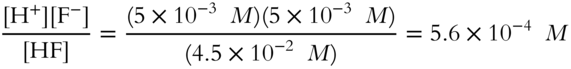
![]() Some bases are also weak electrolytes. The ionization constants of weak bases are calculated in the same way as those of weak acids. An ammonium hydroxide solution is 0.05 M. Assume that the ammonium hydroxide is 2% ionized (dissociated).
Some bases are also weak electrolytes. The ionization constants of weak bases are calculated in the same way as those of weak acids. An ammonium hydroxide solution is 0.05 M. Assume that the ammonium hydroxide is 2% ionized (dissociated).
![]()
Determine the concentration of ![]() ions and OH− ions in the solution. Also determine the concentration of the NH3(aq) that did not dissociate.
ions and OH− ions in the solution. Also determine the concentration of the NH3(aq) that did not dissociate.
[![]() ] = _________
] = _________
[OH−] = __________
[NH3] = __________
Answer: The concentrations of ![]() ions and OH− ions are equal. The solution is 0.05 M but only 2% ionized.
ions and OH− ions are equal. The solution is 0.05 M but only 2% ionized.
![]()
![]()
If 2% is ionized, that leaves 98% in molecular form.
![]()
![]() Determine the value of the ionization constant (Kb) for the base ammonium hydroxide. (Round to the nearest tenth.)
Determine the value of the ionization constant (Kb) for the base ammonium hydroxide. (Round to the nearest tenth.)
![]()
![]()
![]()
Kb = ______________
Answer: 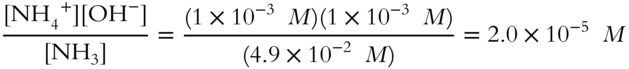
![]() Calculate the ionization constant (Ka) for formic acid (HCOOH) that ionizes (dissociates) 4% in a 0.10 M solution. (Round to the nearest tenth.)
Calculate the ionization constant (Ka) for formic acid (HCOOH) that ionizes (dissociates) 4% in a 0.10 M solution. (Round to the nearest tenth.)
![]()
Ka = ______________
Answer: If 4% is ionized, then 96% must be in molecular form.
![]()
![]()
![]()
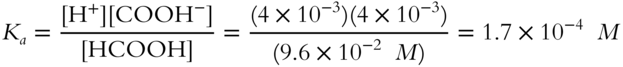
![]() The weak acid H3PO4 is different from previous weak acids because three hydrogen ions are formed for every phosphate (PO43−) ion. An acid with more than one hydrogen ion for each negative ion is called a polyprotic acid. A polyprotic acid loses its hydrogen ions one at a time. The three steps for the dissociation of H3PO4 are:
The weak acid H3PO4 is different from previous weak acids because three hydrogen ions are formed for every phosphate (PO43−) ion. An acid with more than one hydrogen ion for each negative ion is called a polyprotic acid. A polyprotic acid loses its hydrogen ions one at a time. The three steps for the dissociation of H3PO4 are:
1. H3PO4 ⇌ H+ + H2PO4−
2. H2PO4− ⇌ H+ + HPO42−
3. HPO42− ⇌ H+ + PO43−
Write the equilibrium constant expressions for each of these steps.
![]() = __________
= __________
![]() = __________
= __________
![]() = __________
= __________
Answer:
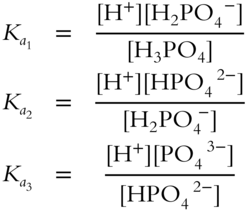
![]() Up to this point, all solutions involving equilibrium have been aqueous. Solvents other than water are also possible since the solvent makes no difference in the calculations. Gases are also possible solutes. One example could be N2O4 in equilibrium with NO2 in a chloroform solvent. Write the equilibrium constant expression (Keq) for N2O4 ⇌ 2NO2.
Up to this point, all solutions involving equilibrium have been aqueous. Solvents other than water are also possible since the solvent makes no difference in the calculations. Gases are also possible solutes. One example could be N2O4 in equilibrium with NO2 in a chloroform solvent. Write the equilibrium constant expression (Keq) for N2O4 ⇌ 2NO2.
Keq = __________
Answer: ![]()
![]() The equilibrium constant (Keq) for the equilibrium of N2O4 ⇌ 2NO2 is equal to 1.1 × 10−5 mol/liter at a specified temperature. A solution of N2O4 in chloroform solvent is determined to be 0.02 mol/liter. Determine the concentration of NO2 at the specified temperature. (Round to the nearest tenth.)
The equilibrium constant (Keq) for the equilibrium of N2O4 ⇌ 2NO2 is equal to 1.1 × 10−5 mol/liter at a specified temperature. A solution of N2O4 in chloroform solvent is determined to be 0.02 mol/liter. Determine the concentration of NO2 at the specified temperature. (Round to the nearest tenth.)
[NO2] = ________________
Answer: The equilibrium expression found in frame 88 can be modified to solve for [NO2].
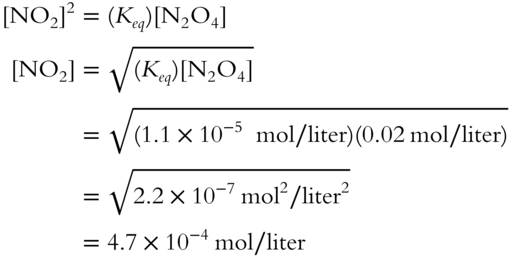
The concepts of chemical and ionic equilibria are very important to you and the chemist. Many useful industrial products, such as NH3 for fertilizers and explosives, require the manipulation of an equilibrium situation to achieve their production. Many physiological processes rely upon equilibria between dissolved salts and weak acids and bases that are present in our bodies. Blood cells must be surrounded by solutions with proper electrolyte balance and H+ ion concentration similar to that found within the cell, or the cell may be damaged. In the next chapter, we say more about acids and bases and their equilibria.
SELF-TEST
This self-test is designed to show how well you have mastered this chapter's objectives. Correct answers and review instructions follow the test.
1. Indicate whether each reaction is a forward or reverse reaction.
1. ![]()
2. ![]()
3. ![]()
4. ![]()
2. Write the equilibrium constant expressions for the following reversible reactions.
1. ![]()
2. ![]()
3. Write the equilibrium constant expressions for the following reversible reactions.
1. ![]()
2. ![]()
4. Write the equilibrium constant expression for the following reaction.
![]()
1. Keq = __________
2. What units will Keq have? __________
5. Calculate how many moles of HI would be present at equilibrium when [H2] = 1 × 10−2, [I2] = 2.5 × 10−2, and Keq = 50 for this equilibrium mixture. Assume system volume is 1 liter. (Round to the nearest hundredth.) __________
![]()
6. Write the equilibrium constant expression for the following equilibrium reaction.
![]()
Keq = ______________________________________
7. Write the equilibrium constant expression for the following equilibrium reaction.
![]()
Keq = ______________________________________
8. Write the equilibrium constant expression for the following equilibrium reaction. Al2Cl6(aq) + 12H2O(l) ⇌ 2Al(H2O)63+(aq) + 6Cl—(aq)
Keq = ____________
9. In what direction will the equilibrium be shifted (left toward the reactant side or right toward the product side) by the following changes?
![]()
1. an increase in pressure __________
2. a decrease in temperature __________
3. a decrease in N2 concentration __________
10. In what direction will the equilibrium be shifted (left towards the reactant side or right toward the product side) by the following changes?
![]()
1. An increase in the concentration H+______________________________
2. A decrease in the concentration of HCO2H______________________________
3. A decrease in the concentration of HCO2—______________________________
11. In what direction will the equilibrium be shifted (left towards the reactant side or right toward the product side) by the following changes?
![]()
1. A decrease in temperature______________________________
2. An increase in the concentration of NH3______________________________
3. A decrease in the concentration of HCl______________________________
12. Calculate (to the nearest hundredth) the molar solubility of BaSO4 if
![]()
13. Calculate (to the nearest hundredth) the molar solubility of PbS if Ksp of PbS = 9.04 x 10—29.
PbS(s) ![]() Pb2+(aq) + S2—(aq)
Pb2+(aq) + S2—(aq)
14. A 0.10 M solution of ammonium hydroxide is found to be 1% ionized. Calculate Kb (to the nearest tenth). __________
15. A 0.15 M solution of hydrocyanic acid (HCN) is found to be 1% ionized. Calculate Ka (to the nearest tenth). The Ka for HCN is 4.9 x 10—10.
ANSWERS
Compare your answers to the self-test with those given below. If you answer all questions correctly, you are ready to proceed to the next chapter. If you miss any, review the frames indicated in parentheses following the answers. If you miss several questions, you should probably reread the chapter carefully.
1.
1. Forward
2. Reverse
3. Forward
4. Reverse [frames 1-5]
2.
1. Keq = ([H2][I2]) / [HI]2[frames 8-13]
2. Keq = [SO3]2 / ([SO2]2[O2]) [frames 8-13]
3.
1. Keq = [NO]2 / ([N2][O2]) [frames 8-13]
2. Keq = [NO2]2 / ([NO]2[O2]) [frames 8-13]
4.
1. 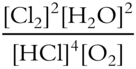 (frames 9−13)
(frames 9−13)
2. liters/mol (frames 18−22)
5.
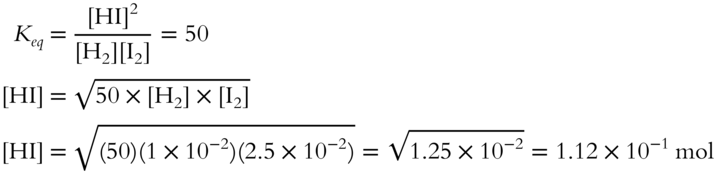
(frames 14−16)
6. Keq = [Pb2+][ I—]2 [frames 9-13, 42—47]
7. ([CH3CO2—][H+]) / [CH3CO2H] [frames 9-13, 42—47]
8. 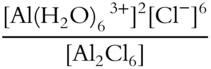 (frames 9−13, 25−30)
(frames 9−13, 25−30)
9.
1. no change (frames 48—51)
2. left toward the reactant side (frames 42—47)
3. left toward the reactant side (frames 34—41)
10.
1. Left toward the reactant side [frames 53-58]
2. Left toward the reactant side [frames 53-58]
3. Right toward the product side [frames 53-58]
11.
1. Right toward the product side [frames 42-47]
2. Right toward the product side [frames 42-47]
3. Left toward the reactant side [frames 42-47]
12.
![]()
if S = molar solubility
![]()
![]()
![]()
![]()
![]()
13. PbS(s) ![]() Pb2+(aq) + S2—(aq)
Pb2+(aq) + S2—(aq)
If S = molar solubility, [Pb2+] = S and [S2—] = S
Ksp = [Pb2+][ S2—] = 9.04 x 10—29
S2 = 9.04 x 10—29
S = 9.51 x 10—15 mol/liter [frames 55, 64, 65]
14.
![]()
![]()
![]()
![]()
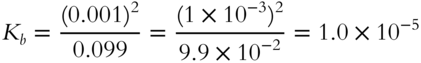
(frames 84, 85)
15. HCN(aq) ![]() CN—(aq) + H+(aq)
CN—(aq) + H+(aq)
[CN—] = (0.15)(0.01) = 0.0015
[H+] = (0.15)(0.01) = 0.0015
[HCN] = (0.15 — 0.0015) = 0.1485
Ka = ([CN—][H+])/[HCN] = (0.0015)2 / 0.1485 = 1.5 x 10—5 [frames 84-86]
EVERYDAY CHEMISTRY
Neutralizing battery corrosion
A friend had purchased a measuring device that used an internal “D” cell battery. The cell had been left in the device too long. The electrolyte had leaked out of the battery and corroded the contacts and part of the inside of the measuring device. The friend had asked for a suggestion to neutralize the corrosion chemical. He did not know whether the “D” cell battery, which had been removed before he purchased it, was an alkaline cell or a low drain carbon zinc cell often labeled as a “heavy duty” cell. We suggested starting with a small amount of vinegar or lemon juice followed by a baking soda mixture (sodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate) and, of course, protecting eyes and skin from any possible contact. The electrolyte in an alkaline cell is KOH, a strong base. The electrolyte in a “heavy duty” carbon zinc cell, meant for low-drain devices, is an acidic mixture typically containing ammonium chloride and zinc chloride.
The friend asked why we had suggested baking soda. We told him that baking soda (NaHCO3) is amphiprotic in an aqueous solution which means it can neutralize either an acidic substance or a base. If the corrosion had been from an alkaline battery, the vinegar which is dilute acetic acid would help neutralize the corrosion. If the corrosion had been from a carbon zinc cell, the vinegar might have added somewhat to the acidic substance but then be quickly neutralized by the baking soda slurry. The “amphi-” part of the word means “both” or “on both sides”. In the next chapter you will learn about the “-protic” part of the word amphiprotic and why it is called that, about something called a “buffer” solution, and about two chemists named Brønsted and Lowry who were independently responsible for a related theory of acids and bases.