Chemistry: A Self-Teaching Guide - Post R., Snyder C., Houk C.C. 2020
Gases
Up to this point you have dealt with the states of matter (gases, liquids, and solids) when they appear as reactants or products in chemical reactions, but we have not discussed their properties.
In this chapter we discuss the properties of gases, how they behave, and how they are affected by changes in pressure and temperature. Several new laws will be introduced that deal with the behavior and properties of gases. You will also see how the molecular weights of gases are obtained from experimental measurements of the weights and volumes of samples of gaseous substances. It is possible to determine the molecular weight of any gas using the laws that are presented because all gases respond in much the same manner to changes in temperature and pressure.
As you work through this chapter we suggest that you think about encounters you have had with gases, such as a bicycle pump, a flat tire, a balloon (filled with air or helium), and the odors of ammonia, perfumes, or skunks. When dealing with problems involving gases, remember to use your experience and common sense.
OBJECTIVES
After completing this chapter, you will be able to
· recognize and apply or illustrate: pressure, temperature, partial pressure, kinetic molecular theory, diffusion, effusion, standard temperature and pressure (STP), Boyle's Law, Charles's Law, Dalton's Law, Graham's Law, combined gas law equation, ideal gas law, absolute temperature, absolute zero, and compressibility;
· calculate the fourth term in Boyle's or Charles's Laws when given any three of the four terms in the equations;
· calculate the sixth term in the combined gas law equation when given any five of the six terms in the equation;
· calculate the molecular weight of any gas when given
1. its density (two ways: using the volume per mole of a gas at STP or Graham's Law);
2. the rate of effusion of the gas compared to the rate of another gas (Graham's Law);
3. P, V, R, T, and the weight of a sample of the gas (using the ideal gas law);
· calculate the weight of a liter of a gas or the volume occupied by 1 gram of a gas when given its molecular formula;
· state the assumptions of the kinetic molecular theory of gases and how they relate to ideal and real gases;
· calculate, using Dalton's Law,
1. the partial pressure exerted by each gas within a mixture of gases when given the total pressure of the mixture and the mole fraction of each gas in the mixture (or the data necessary to calculate the mole fractions) and vice versa;
2. the volume percent of each gas in a mixture when given the total volume of gases and the mole fraction of each gas (or the data needed to calculate the mole fraction) and vice versa.
KINETIC MOLECULAR THEORY
![]() The next three chapters will deal with the three states of matter: gases, liquids, and solids. These three states are related. If a solid is heated sufficiently, it will melt and become a liquid. If a liquid is heated sufficiently, it will become a gas or vapor. If a gas is cooled sufficiently, it will liquefy, and if a liquid is cooled sufficiently, it will solidify.
The next three chapters will deal with the three states of matter: gases, liquids, and solids. These three states are related. If a solid is heated sufficiently, it will melt and become a liquid. If a liquid is heated sufficiently, it will become a gas or vapor. If a gas is cooled sufficiently, it will liquefy, and if a liquid is cooled sufficiently, it will solidify.
Gases are defined on the basis of the kinetic molecular theory. This theory makes several assumptions about gases. The first assumption is that gases are made up of individual molecules that are in constant rapid motion. The molecules possess kinetic energy (energy of motion) and bounce into each other and the walls of the gas container. Molecules bump into the wall at the top of a container as often as they bump into the bottom. This contrasts with the molecules of a liquid, which can flow and glide over one another but are, as you know, kept toward the bottom of the container. For example, the liquid molecules of a glass of water stay at the bottom of the glass. In a solid, the molecules can vibrate and bend, but they cannot flow or glide over one another or bounce around freely.
The kinetic molecular theory also assumes that the molecules of a gas are much farther apart than molecules of a liquid or solid.
1. In which state do molecules have the most freedom to move about? ________________
2. Which do you expect would contain more molecules, a liter of a gas such as oxygen or a liter of a liquid such as water? ________________
Answer:
1. gas (Gas molecules can move rapidly in any direction. Molecules of a liquid or solid are more restricted.)
2. a liter of liquid (In a gas, the molecules are farther apart than in a liquid. Therefore, there would be fewer molecules in a given volume of a gas such as a liter.)
![]() The constantly moving gas molecules possess kinetic energy (energy of motion). All gases at the same temperature are assumed to have the same average kinetic energy. In fact, temperature is simply a measure of the average kinetic energy. Heating a gas results in an increase in the temperature of the gas and an increase in the average kinetic energy of gas molecules.
The constantly moving gas molecules possess kinetic energy (energy of motion). All gases at the same temperature are assumed to have the same average kinetic energy. In fact, temperature is simply a measure of the average kinetic energy. Heating a gas results in an increase in the temperature of the gas and an increase in the average kinetic energy of gas molecules.
The average kinetic energy of sample A of a gas is greater than that of sample B of that gas. Which gas sample (A or B) could be expected to have a higher temperature? (All other conditions are equal.) _______________
Answer: sample A (At constant temperature, all gases are assumed to have the same kinetic energy. Therefore the gas with a higher kinetic energy would also be of higher temperature.)
![]() The kinetic molecular theory assumes that the rapidly moving gas molecules collide with each other and the walls of the gas container without any loss of kinetic energy. The collision of the gas molecules with the walls of the gas container results in what we call pressure.
The kinetic molecular theory assumes that the rapidly moving gas molecules collide with each other and the walls of the gas container without any loss of kinetic energy. The collision of the gas molecules with the walls of the gas container results in what we call pressure.
A balloon is kept inflated because of pressure caused by ________________.
Answer: the collision of gas molecules with the inside wall of the balloon
![]() Another assumption of the kinetic molecular theory is that the gas molecules themselves occupy no volume (thus leaving only the space between molecules as the volume occupied by a gas) and have no attraction for each other. Note that a gas that behaves completely according to the kinetic molecular theory assumptions is a theoretically ideal gas. Most actual gases approximate an ideal gas very well at moderate temperatures and pressures because of the large amount of space between the molecules. At low temperatures and high pressures, normal gases deviate from a theoretically ideal gas. We will cover some of these deviations later. Calculations involving pressure, volume, and temperature of gases are much simpler if we assume that gases behave as theoretically ideal. According to the kinetic molecular theory, all of the volume of an ideal gas can be attributed to ___________________________________________________.
Another assumption of the kinetic molecular theory is that the gas molecules themselves occupy no volume (thus leaving only the space between molecules as the volume occupied by a gas) and have no attraction for each other. Note that a gas that behaves completely according to the kinetic molecular theory assumptions is a theoretically ideal gas. Most actual gases approximate an ideal gas very well at moderate temperatures and pressures because of the large amount of space between the molecules. At low temperatures and high pressures, normal gases deviate from a theoretically ideal gas. We will cover some of these deviations later. Calculations involving pressure, volume, and temperature of gases are much simpler if we assume that gases behave as theoretically ideal. According to the kinetic molecular theory, all of the volume of an ideal gas can be attributed to ___________________________________________________.
Answer: the space between the molecules
![]() Gases can be more readily compressed than liquids or solids. Since gas molecules are relatively far apart, an external force or pressure can readily push the molecules closer together.
Gases can be more readily compressed than liquids or solids. Since gas molecules are relatively far apart, an external force or pressure can readily push the molecules closer together.
If a certain volume (such as a liter) of gas is compressed to half that volume, what happens to the number of molecules of gas during compression? (increases, decreases, stays the same) _____________________
Answer: stays the same (Just the space between the molecules is decreased.)
![]() Besides greater compressibility than liquids or solids, a characteristic of gases is greater diffusibility than liquids or solids. One gas rapidly diffuses into (mixes completely with) another gas because the space between gas molecules allows ready access to molecules of another gas.
Besides greater compressibility than liquids or solids, a characteristic of gases is greater diffusibility than liquids or solids. One gas rapidly diffuses into (mixes completely with) another gas because the space between gas molecules allows ready access to molecules of another gas.
Why could you expect gases to diffuse more rapidly than liquids under roughly comparable conditions? ______________________
Answer: The space between molecules of a gas is greater than the space between molecules of a comparable liquid.
![]() When a gas is compressed, the same number of gas molecules occupies a smaller volume than before compression. That is, the gas container is smaller but contains the same number of molecules. We have defined pressure as the collision of gas molecules with the wall of the gas container. When a gas is compressed would you expect an increase, decrease, or no change in the number of collisions of gas molecules per square unit (such as a square centimeter) of container wall? _____________ Why? _______________________________________
When a gas is compressed, the same number of gas molecules occupies a smaller volume than before compression. That is, the gas container is smaller but contains the same number of molecules. We have defined pressure as the collision of gas molecules with the wall of the gas container. When a gas is compressed would you expect an increase, decrease, or no change in the number of collisions of gas molecules per square unit (such as a square centimeter) of container wall? _____________ Why? _______________________________________
Answer: an increase (There are just as many gas molecules now striking the wall of the smaller container as were striking the wall of the larger container. However, the container is now smaller; therefore, the number of collisions per square unit of container wall surface has increased.)
![]() Pressure is often measured as the relative number of collisions on a given area (square unit) of the gas container. Would you expect the pressure of a compressed gas to be greater than, less than, or the same as that of the gas before compression? _________________________
Pressure is often measured as the relative number of collisions on a given area (square unit) of the gas container. Would you expect the pressure of a compressed gas to be greater than, less than, or the same as that of the gas before compression? _________________________
Answer: greater (Under compression, more gas molecules collide on a given area of the gas container.)
![]() Answer these questions about the effect of pressure on a gas.
Answer these questions about the effect of pressure on a gas.
1. At constant temperature, when the volume of a gas (with a specific and unchanging number of molecules) is decreased by compression, the pressure on a given area of the inside wall of the container is (decreased, increased, unchanged) ________________
2. At constant temperature (with a specific number of gas molecules), when the pressure of a gas increases, the volume of the gas is probably being (increased, decreased) __________________
Answer: (a) increased; (b) decreased
BOYLE'S LAW
![]() A scientist named Boyle formalized the relationship between pressure and volume that you have just seen in the previous frame. Boyle's Law states that at constant temperature, the volume of a confined dry gas is inversely proportional to the pressure. (Dry indicates no water present.) Mathematically, Boyle's Law is stated as:
A scientist named Boyle formalized the relationship between pressure and volume that you have just seen in the previous frame. Boyle's Law states that at constant temperature, the volume of a confined dry gas is inversely proportional to the pressure. (Dry indicates no water present.) Mathematically, Boyle's Law is stated as:
![]()
Pressure and volume are inversely proportional. Mathematically this means that if we multiply pressure by some number, we must divide the volume by that same number, or vice versa. For example, assume the constant is equal to the number 4. Assume that the pressure and the volume each are equal to 2:
![]()
If we divide the pressure by 2, we must multiply the volume by 2 in order for the constant to remain unchanged:
![]()
If we multiply the pressure by 10, we must divide the volume by 10 in order for the constant to remain unchanged:
![]()
At constant temperature with a confined specific amount of gas molecules: (a) if the volume is decreased, the pressure is __________ and (b) if the pressure is increased, the volume is _________________.
Answer: (a) increased; (b) decreased
![]() The pressure (P) multiplied by the volume (V) is a constant (unchanging) number if the temperature remains constant. Note that Boyle's Law is based on a theoretically ideal gas. Real gases behave in a similar fashion to an ideal gas at moderate temperatures and pressures. Unless otherwise stated, we will assume that all gases behave exactly like an ideal gas. Since all gases are assumed to behave ideally, one gas will behave exactly like any other gas.
The pressure (P) multiplied by the volume (V) is a constant (unchanging) number if the temperature remains constant. Note that Boyle's Law is based on a theoretically ideal gas. Real gases behave in a similar fashion to an ideal gas at moderate temperatures and pressures. Unless otherwise stated, we will assume that all gases behave exactly like an ideal gas. Since all gases are assumed to behave ideally, one gas will behave exactly like any other gas.
According to the equation for Boyle's Law at constant temperature:
1. increasing P causes a corresponding ______________ in V.
2. increasing V causes a corresponding _______________ in P.
Answer: (a) decrease; (b) decrease
![]() Boyle's Law can be applied in practical situations involving the pressure and volume of a gas at constant temperature. A gas originally occupies a volume, which can be called V1, at the pressure of P1. By Boyle's Law, we know that multiplying P1 times V1 gives a number that is a constant. This same gas undergoes an increase in pressure to P2 and a corresponding decrease in volume to V2. There is no change in temperature. How does the numerical constant obtained by multiplying P1 by V1 compare with the numerical constant obtained by multiplying P2 by V2? ________________________________________
Boyle's Law can be applied in practical situations involving the pressure and volume of a gas at constant temperature. A gas originally occupies a volume, which can be called V1, at the pressure of P1. By Boyle's Law, we know that multiplying P1 times V1 gives a number that is a constant. This same gas undergoes an increase in pressure to P2 and a corresponding decrease in volume to V2. There is no change in temperature. How does the numerical constant obtained by multiplying P1 by V1 compare with the numerical constant obtained by multiplying P2 by V2? ________________________________________
Answer: The numerical constants are exactly the same. (A constant does not change.)
![]() P1V1 is equal to a constant and if P2V2 is equal to that same constant, then P1V1 must equal P2V2. This is a more practical expression of Boyle's Law. If the new pressure (P2) of a quantity of gas is greater than the earlier pressure (P1) of that gas, and the temperature is assumed to be constant, then the new volume (V2) must be (greater, less) ____________ than the earlier volume (V1).
P1V1 is equal to a constant and if P2V2 is equal to that same constant, then P1V1 must equal P2V2. This is a more practical expression of Boyle's Law. If the new pressure (P2) of a quantity of gas is greater than the earlier pressure (P1) of that gas, and the temperature is assumed to be constant, then the new volume (V2) must be (greater, less) ____________ than the earlier volume (V1).
Answer: less (Pressure and volume are inversely proportional. As one increases, the other decreases.)
![]() The equation P1V1 = P2V2 has four unknowns. If any three quantities are known, then the fourth can be determined. If P1 is the unknown, the expression can be written as
The equation P1V1 = P2V2 has four unknowns. If any three quantities are known, then the fourth can be determined. If P1 is the unknown, the expression can be written as
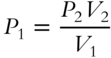
by simply dividing both sides of the equation by V1. Suppose that V2 is unknown and P1, P2, and V1 are known. Rewrite the equation P1V1 = P2V2 so that only V2 remains on one side of the equation.
Answer: 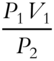 (Divide both sides of the equation by P2.)
(Divide both sides of the equation by P2.)
![]() V1 is the original volume and P1 is the original pressure. V2 is the new volume and I2 is the new pressure. The pressure of a gas is measured in the metric system as millimeters of mercury. In honor of Torricelli, who did pioneering work with pressure, a new unit was accepted. A pressure that will support a column of mercury 1 millimeter high is called a torr. Millimeters of mercury and torr are equivalent and interchangeable as measures of pressure. Some textbooks use millimeters of mercury; we will use torr. The volume of a gas is usually designated in liters or milliliters.
V1 is the original volume and P1 is the original pressure. V2 is the new volume and I2 is the new pressure. The pressure of a gas is measured in the metric system as millimeters of mercury. In honor of Torricelli, who did pioneering work with pressure, a new unit was accepted. A pressure that will support a column of mercury 1 millimeter high is called a torr. Millimeters of mercury and torr are equivalent and interchangeable as measures of pressure. Some textbooks use millimeters of mercury; we will use torr. The volume of a gas is usually designated in liters or milliliters.
Five liters of a gas exist at a pressure of 700 torr. If the pressure is increased to 1400 torr, the volume of the gas is decreased. Let V2 represent the unknown new volume. Identify V1, P1, and P2 in this example.
V1 = ______________
P1 = ______________
P2 = ______________
Answer: 5 liters; 700 torr; 1400 torr
![]() Using the information from frame 15, determine the new volume (V2). (Temperature is constant.)
Using the information from frame 15, determine the new volume (V2). (Temperature is constant.)
V2 = __________________________________________________________
Answer: 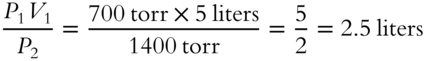
(Remember to express the volume in liters.)
![]() Five liters of oxygen gas exist at a pressure of 700 torr. If the pressure is decreased to 600 torr and temperature is constant, what will be the new volume? (If rounding is required, round to the nearest hundredth for this answer and others unless otherwise indicated.)
Five liters of oxygen gas exist at a pressure of 700 torr. If the pressure is decreased to 600 torr and temperature is constant, what will be the new volume? (If rounding is required, round to the nearest hundredth for this answer and others unless otherwise indicated.)
V2 = _____________
Answer: 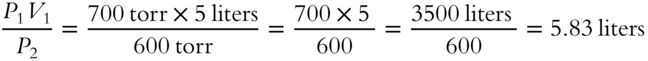
![]() Five liters of oxygen gas exist at a pressure of 700 torr. The pressure of the gas is reduced to a quarter of the original pressure. (A quarter of 700 torr pressure is
Five liters of oxygen gas exist at a pressure of 700 torr. The pressure of the gas is reduced to a quarter of the original pressure. (A quarter of 700 torr pressure is ![]() .) The temperature is constant. What is the new volume of oxygen?
.) The temperature is constant. What is the new volume of oxygen?
V2 = _____________
Answer: 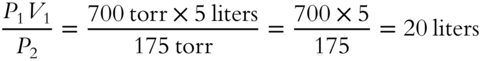
![]() Assume the temperature does not change. Pressure and volume are inversely proportional. If a quantity of gas is compressed to half its volume, the new pressure will be doubled. If a quantity of gas is compressed to
Assume the temperature does not change. Pressure and volume are inversely proportional. If a quantity of gas is compressed to half its volume, the new pressure will be doubled. If a quantity of gas is compressed to ![]() of its volume, its new pressure must be how many times as great as that of the original pressure? (If you wish to do so, try this problem on the 5 liters of gas at the pressure of 700 torr. The new volume would be 0.5 liters.) What is the new pressure in relation to the original pressure?
of its volume, its new pressure must be how many times as great as that of the original pressure? (If you wish to do so, try this problem on the 5 liters of gas at the pressure of 700 torr. The new volume would be 0.5 liters.) What is the new pressure in relation to the original pressure?
P2 = _____________
Answer: 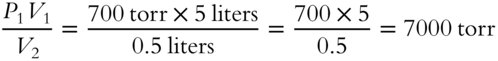
The new pressure is 10 times the original pressure.
![]() A pressure of 760 torr or millimeters of mercury is equivalent to 1 atm (atmosphere) of pressure. One atmosphere is the average pressure produced by the earth's atmosphere at sea level. A pressure of 1 atm supports a column of mercury that is 760 millimeters high. Ten liters of hydrogen gas at a pressure of 1 atm is compressed to a new pressure of 850 torr. What is the new volume of the gas?
A pressure of 760 torr or millimeters of mercury is equivalent to 1 atm (atmosphere) of pressure. One atmosphere is the average pressure produced by the earth's atmosphere at sea level. A pressure of 1 atm supports a column of mercury that is 760 millimeters high. Ten liters of hydrogen gas at a pressure of 1 atm is compressed to a new pressure of 850 torr. What is the new volume of the gas?
V2 = _____________
Answer: 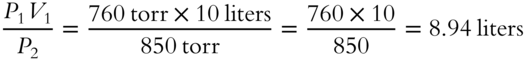
![]() Another unit for pressure is the kilopascal. One atmosphere is equal to 760 torr, which is equal to 101.325 kilopascals (kPa). A quantity of gas with an original pressure of 100 kPa is compressed to 150 kPa. The new volume of gas is 2 liters. What was the original volume of gas?
Another unit for pressure is the kilopascal. One atmosphere is equal to 760 torr, which is equal to 101.325 kilopascals (kPa). A quantity of gas with an original pressure of 100 kPa is compressed to 150 kPa. The new volume of gas is 2 liters. What was the original volume of gas?
V1 = _________________________________________________________
Answer: 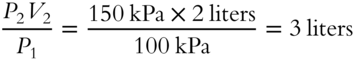
![]() An aerosol spray can has a volume of 0.15 liters. It is designed so that it can deliver 1000 times its original volume to a new pressure assumed to be equal to one atmosphere. What is the original pressure inside the can? (An answer in terms of either torr or atmospheres (atm) is acceptable.)
An aerosol spray can has a volume of 0.15 liters. It is designed so that it can deliver 1000 times its original volume to a new pressure assumed to be equal to one atmosphere. What is the original pressure inside the can? (An answer in terms of either torr or atmospheres (atm) is acceptable.)
P1 = __________________________________________________________
Answer: There are several ways to solve this problem. The first two methods use the fact that 1000 times the original 0.15 liter volume is 1000 × 0.15 = 150 liters.
· Method 1: 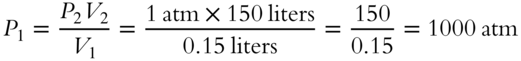
· Method 2: 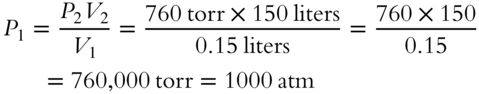
· Method 3: Merely knowing that the new volume is 1000 times greater than the original volume is useful. The new volume (V2) equals 1000 × V1. Substitute (1000V1) for V2.
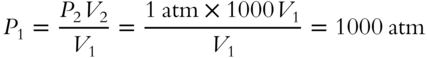
![]() According to Boyle's Law, the pressure times the volume of a gas is a constant at constant temperature. Remember that all gases at a constant temperature are assumed to have the same average kinetic energy. Temperature is simply a measurement of the average kinetic energy of the gas.
According to Boyle's Law, the pressure times the volume of a gas is a constant at constant temperature. Remember that all gases at a constant temperature are assumed to have the same average kinetic energy. Temperature is simply a measurement of the average kinetic energy of the gas.
Based on these statements, would you expect the average kinetic energy of a gas to remain the same if the temperature was changed? ________________
Answer: No, a change in temperature indicates a change in the average kinetic energy.
![]() The absolute temperature scale is a measure of the average kinetic energy of gases. Doubling the absolute temperature doubles the average kinetic energy. Doubling the kinetic energy results in doubling the pressure (twice as many collisions of gas molecules with the walls) if the volume is constant, or a doubling of the volume if the pressure is constant. What happens to the average kinetic energy of a gas as the absolute temperature increases? ____________________
The absolute temperature scale is a measure of the average kinetic energy of gases. Doubling the absolute temperature doubles the average kinetic energy. Doubling the kinetic energy results in doubling the pressure (twice as many collisions of gas molecules with the walls) if the volume is constant, or a doubling of the volume if the pressure is constant. What happens to the average kinetic energy of a gas as the absolute temperature increases? ____________________
Answer: The average kinetic energy increases.
CHARLES'S LAW
![]() With the pressure constant, the volume of a gas is directly proportional to the temperature. (That is, if the volume is multiplied by a number, the temperature must be multiplied by that same number. If the volume is divided by some number, the temperature must also be divided by that number.)
With the pressure constant, the volume of a gas is directly proportional to the temperature. (That is, if the volume is multiplied by a number, the temperature must be multiplied by that same number. If the volume is divided by some number, the temperature must also be divided by that number.)
A scientist named Charles first stated this relationship formally. Charles's Law can be stated mathematically as:
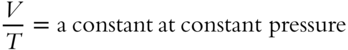
V represents the volume of a gas and T is the absolute temperature of a gas.
1. In Boyle's Law, at constant temperature, the pressure and volume are (directly, inversely) ________________ proportional.
2. In Charles's Law, at constant pressure, the volume and absolute temperature are (directly, inversely) ________________ proportional.
Answer: (a) inversely; (b) directly
![]() Charles's Law can be rewritten as:
Charles's Law can be rewritten as:

V1 is the original volume and V2 is the new volume. T1 is the original temperature and T2 is the new temperature.
A quantity of gas at 200 K (absolute temperature scale) is heated to 400 K. The original volume is 3 liters. What is the new volume (with no change in pressure)?____________________________________________
Answer: Charles's Law applies here. Modify the equation so that just V2 is on the left side of the equation by multiplying both sides by T2.
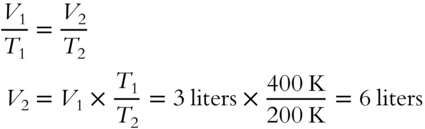
![]() Charles's Law is based on absolute temperature. The absolute (Kelvin) temperature scale is comparable to the Celsius scale in size of the degree, but the zero point is different. The zero point for the Kelvin scale is known as absolute zero. The zero point for the Celsius scale is the freezing point of water. The Kelvin scale zero point (0 K) is equivalent to −273°C. The freezing point of water is 0°C, or 273 K. To change from Celsius to Kelvin, just add 273 to the Celsius temperature. To change from Kelvin to Celsius, just subtract 273 from the Kelvin temperature. Fill in the blanks in the following conversions.
Charles's Law is based on absolute temperature. The absolute (Kelvin) temperature scale is comparable to the Celsius scale in size of the degree, but the zero point is different. The zero point for the Kelvin scale is known as absolute zero. The zero point for the Celsius scale is the freezing point of water. The Kelvin scale zero point (0 K) is equivalent to −273°C. The freezing point of water is 0°C, or 273 K. To change from Celsius to Kelvin, just add 273 to the Celsius temperature. To change from Kelvin to Celsius, just subtract 273 from the Kelvin temperature. Fill in the blanks in the following conversions.
0°C = 273 K
10°C = 283 K
50°C = ___________K
_______ = 300 K
Answer: 323; 27°C
![]() At absolute zero, an ideal gas has theoretically no volume at all. A gas loses volume at the rate of 1/273 for every degree of temperature drop at constant pressure. The temperature at which a theoretically ideal gas has no volume is ____________ °Celsius.
At absolute zero, an ideal gas has theoretically no volume at all. A gas loses volume at the rate of 1/273 for every degree of temperature drop at constant pressure. The temperature at which a theoretically ideal gas has no volume is ____________ °Celsius.
Answer: −273
![]() A quantity of gas at 10°C and 2 liters volume is heated to 50°C. Determine the new volume if the pressure remains constant. (Hint: To make calculations simpler when using Charles's Law, change all temperatures to the absolute, or Kelvin, scale first.)
A quantity of gas at 10°C and 2 liters volume is heated to 50°C. Determine the new volume if the pressure remains constant. (Hint: To make calculations simpler when using Charles's Law, change all temperatures to the absolute, or Kelvin, scale first.)
Answer: Charles's Law applies here.
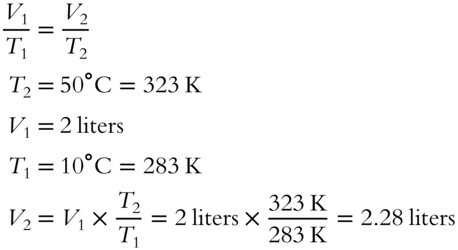
![]() A sample of neon gas occupies 100 milliliters (mL) volume at 100°C. The sample is heated to 200°C. What is the new volume (to the nearest tenth) of neon (pressure unchanged)? _________________
A sample of neon gas occupies 100 milliliters (mL) volume at 100°C. The sample is heated to 200°C. What is the new volume (to the nearest tenth) of neon (pressure unchanged)? _________________
Answer:
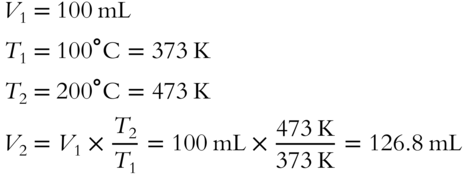
![]() A sample of chlorine gas occupies 100 mL at −11°C. Heating (with no increase in pressure) changes its volume to 150 mL.
A sample of chlorine gas occupies 100 mL at −11°C. Heating (with no increase in pressure) changes its volume to 150 mL.
1. What is the new temperature as expressed on the absolute temperature scale? _________
2. Express T2 in Celsius degrees. ______________
Answer:
1.
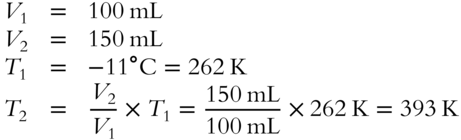
2. 120°C
PARTIAL PRESSURE
![]() When two or more different gases are mixed together, each gas in the mixture contributes to the total pressure of the mixture. The pressure contributed by each gas is in direct proportion to the number of molecules of the gas in the mixture. In a particular mixture of nitrogen gas and oxygen gas, there are twice as many nitrogen molecules as oxygen molecules. Each gas molecule contributes equal amounts to the total pressure regardless of what kind of molecule it is. Since there are twice as many nitrogen gas molecules as oxygen molecules in this mixture, we could expect the nitrogen molecules to contribute (half as much, twice as much, an equal amount) _______________ to the total pressure as the oxygen molecules.
When two or more different gases are mixed together, each gas in the mixture contributes to the total pressure of the mixture. The pressure contributed by each gas is in direct proportion to the number of molecules of the gas in the mixture. In a particular mixture of nitrogen gas and oxygen gas, there are twice as many nitrogen molecules as oxygen molecules. Each gas molecule contributes equal amounts to the total pressure regardless of what kind of molecule it is. Since there are twice as many nitrogen gas molecules as oxygen molecules in this mixture, we could expect the nitrogen molecules to contribute (half as much, twice as much, an equal amount) _______________ to the total pressure as the oxygen molecules.
Answer: twice as much (The pressure contribution of a particular gas to the total pressure of a gas mixture is directly proportional to the relative number of molecules of the gas in the mixture. Twice as many molecules cause twice as much pressure contribution.)
![]() In a gas mixture of nitrogen and oxygen, there are twice as many nitrogen molecules as oxygen molecules. One-third of the total pressure of the mixture is contributed by the oxygen molecules; two-thirds is contributed by the nitrogen molecules. The total pressure of the gas mixture is 900 torr.
In a gas mixture of nitrogen and oxygen, there are twice as many nitrogen molecules as oxygen molecules. One-third of the total pressure of the mixture is contributed by the oxygen molecules; two-thirds is contributed by the nitrogen molecules. The total pressure of the gas mixture is 900 torr.
1. Nitrogen contributes how much to the total pressure? __________ torr
2. Oxygen contributes how much to the total pressure? __________ torr
Answer: (a) 600 (Twice as much, or two-thirds of the total, is contributed by nitrogen: ![]() ); (b)
); (b) ![]()
![]() The total pressure of a mixture of gas is the sum of all partial pressures. The partial pressures are the pressures contributed by each of the gases. A partial pressure of a particular gas is proportional to the amount (relative number of molecules) of the gas within the mixture. In the previous example, 600 torr is the partial pressure exerted by nitrogen gas, 300 torr is the partial pressure of the oxygen gas, and 900 torr is the total pressure, which is the sum of all partial pressures in the mixture.
The total pressure of a mixture of gas is the sum of all partial pressures. The partial pressures are the pressures contributed by each of the gases. A partial pressure of a particular gas is proportional to the amount (relative number of molecules) of the gas within the mixture. In the previous example, 600 torr is the partial pressure exerted by nitrogen gas, 300 torr is the partial pressure of the oxygen gas, and 900 torr is the total pressure, which is the sum of all partial pressures in the mixture.
Dalton's Law of Partial Pressures (named after its discoverer) states that the total pressure of a mixture of gases is the sum of all of the partial pressures of the individual gases. The molecules of each gas exert the same pressure within the mixture as they would if they were not in the mixture. Dalton's Law can be written mathematically as:
![]()
The PT (in capital letters) represents the total pressure. What do p1, p2, p3, and so on (in lowercase letters) represent? ____________________________
Answer: partial pressures
![]() Determining partial pressures of gases is useful when analyzing gas mixtures. In order to determine the partial pressures of the individual gases in a mixture, we must first know the relative number of molecules of each gas in the mixture. Since a molecule is a very small unit, it is easier to use the mole. Partial pressures are proportional to the number of moles of the individual gases in a mixture. Partial pressure can be determined by dividing the moles of each particular gas by the total gas moles in the mixture and multiplying the result by the total pressure. A mixture of argon and xenon gases involves 2 mol of argon and 5 mol of xenon. The total number of moles of the mixture is 2 + 5 = 7 mol. The total pressure exerted by the mixture is 700 torr. The total pressure exerted by argon is 2/7 mol × 700 torr = 200 torr. What is the partial pressure exerted by xenon? (Remember that according to Dalton's Law the total pressure is equal to the sum of the partial pressures.) ________________________
Determining partial pressures of gases is useful when analyzing gas mixtures. In order to determine the partial pressures of the individual gases in a mixture, we must first know the relative number of molecules of each gas in the mixture. Since a molecule is a very small unit, it is easier to use the mole. Partial pressures are proportional to the number of moles of the individual gases in a mixture. Partial pressure can be determined by dividing the moles of each particular gas by the total gas moles in the mixture and multiplying the result by the total pressure. A mixture of argon and xenon gases involves 2 mol of argon and 5 mol of xenon. The total number of moles of the mixture is 2 + 5 = 7 mol. The total pressure exerted by the mixture is 700 torr. The total pressure exerted by argon is 2/7 mol × 700 torr = 200 torr. What is the partial pressure exerted by xenon? (Remember that according to Dalton's Law the total pressure is equal to the sum of the partial pressures.) ________________________
Answer: 700 torr − 200 torr = 500 torr
![]() We could have solved the problem in frame 35 by using the following formula for partial pressure:
We could have solved the problem in frame 35 by using the following formula for partial pressure:
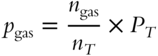
The formula symbols represent the following quantities.

A mixture of gases exerting a pressure of 840 torr consists of 0.2 mol of O2 gas, 0.3 mol of H2 gas, and 0.7 mol of CO2 gas. The pressure contributed by the H2 gas is ____________ torr.
Answer: H2, the gas in question, has 0.3 mol in the mixture. The total moles of gas is 0.2 + 0.3 + 0.7 = 1.2 mol.
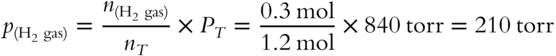
(Because H2 gas accounts for one-quarter of the total moles of gas in the mixture, it accounts for one-quarter of the total pressure.)
![]() In the same gas mixture, the pressure contributed by the CO2 gas is _____ torr.
In the same gas mixture, the pressure contributed by the CO2 gas is _____ torr.
Answer: 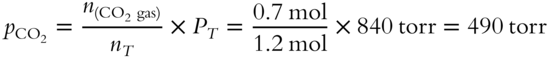
![]() The formula for partial pressure involves multiplying the mole fraction by the total pressure. The mole fraction is simply the number of moles of a particular gas divided by the total number of moles of gas in a mixture:
The formula for partial pressure involves multiplying the mole fraction by the total pressure. The mole fraction is simply the number of moles of a particular gas divided by the total number of moles of gas in a mixture:
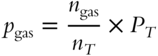
Which part of the above formula represents the mole fraction? _____________
Answer: ![]()
![]() The gas mixture in frame 36 consisted of 0.2 mol of O2, 0.3 mol of H2, and 0.7 mol of CO2. The total gas moles were 0.2 + 0.3 + 0.7 = 1.2 mol. The mole fractions of each of the gas components are as follows.
The gas mixture in frame 36 consisted of 0.2 mol of O2, 0.3 mol of H2, and 0.7 mol of CO2. The total gas moles were 0.2 + 0.3 + 0.7 = 1.2 mol. The mole fractions of each of the gas components are as follows.
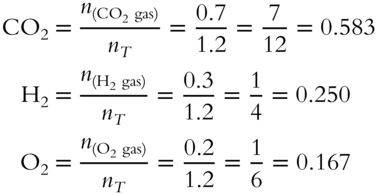
Mole fractions can be expressed in fractional or decimal form. The mole fraction of a gas is the number of moles of a particular gas divided by the total number of moles of gas in a mixture.
1. The mole fraction for CO2 in the above gas mixture is _____________ .
2. The mole fractions of all gases in the mixture add up to _______________.
Answer:
1. ![]() or 0.583
or 0.583
2. 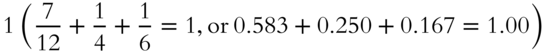
![]() Mole fractions are very useful for determining the percentage volume for each gas in a gas mixture. All mole fractions in a gas mixture add up to 1. If we multiply the individual mole fractions by 100, the results are the percentages by volume of the various gases in the mixture. The previous mixture was made up of 0.250 × 100 = 25.0% hydrogen, 0.167 × 100 = 16.7% oxygen, and 0.583 × 100 = __________% carbon dioxide.
Mole fractions are very useful for determining the percentage volume for each gas in a gas mixture. All mole fractions in a gas mixture add up to 1. If we multiply the individual mole fractions by 100, the results are the percentages by volume of the various gases in the mixture. The previous mixture was made up of 0.250 × 100 = 25.0% hydrogen, 0.167 × 100 = 16.7% oxygen, and 0.583 × 100 = __________% carbon dioxide.
Answer: 58.3
![]() A gas mixture is made up of 25.0% H2 gas, 16.7% O2 gas, and 58.3% CO2 gas. If the total volume of the mixture is 100.0 liters, the mixture could be expected to be made up of how many liters (to the nearest tenth) of H2 gas, CO2 gas, and O2 gas? ___________
A gas mixture is made up of 25.0% H2 gas, 16.7% O2 gas, and 58.3% CO2 gas. If the total volume of the mixture is 100.0 liters, the mixture could be expected to be made up of how many liters (to the nearest tenth) of H2 gas, CO2 gas, and O2 gas? ___________
Answer: 25.0, 16.7, 58.3
![]() A corollary to Dalton's Law of Partial Pressures is the following:
A corollary to Dalton's Law of Partial Pressures is the following:
![]()
The total volume (VT) of a mixture of gases is the sum of all partial volumes (v1, v2, v3, and so on).
A mixture is made up of 25.0 liters of H2, 16.7 liters of O2, and 58.3 liters of CO2. What is VT for the mixture?
Answer: 100 liters (25.0 + 16.7 + 58.3 = 100 liters)
![]() Mole fractions are useful for determining the partial pressure contributed by a particular gas in a mixture or for determining the volume attributable to a gas in a mixture. Mole fractions are particularly useful in chemical analysis of a mixture of gases.
Mole fractions are useful for determining the partial pressure contributed by a particular gas in a mixture or for determining the volume attributable to a gas in a mixture. Mole fractions are particularly useful in chemical analysis of a mixture of gases.
You have already determined mole fractions by dividing the moles of a gas by the total moles in the mixture. A mole fraction can also be determined by dividing the partial pressure of a gas by the total pressure of all gases in the mixture:
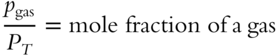
A mole fraction can also be determined by dividing the partial volume of a gas by the total volume of all gases in the mixture:
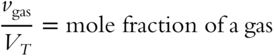
A mixture of gases is made up of 10 mL of neon, 20 mL of xenon, and 70 mL of nitrogen. What is the mole fraction of xenon in the mixture? ____________
Answer: VT = v1 + v2 + v3 = 10 mL + 20 mL + 70 mL = 100 mL
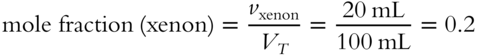
![]() A mixture of three gases (hydrogen, oxygen, and nitrogen) is chemically treated to absorb one gas at a time, using a process called selective absorption. Pressure is kept constant. The total original volume is 12 liters. After the oxygen is removed, the volume is 10 liters. After the hydrogen is removed, 6 liters of gas remain. The mixture contained:
A mixture of three gases (hydrogen, oxygen, and nitrogen) is chemically treated to absorb one gas at a time, using a process called selective absorption. Pressure is kept constant. The total original volume is 12 liters. After the oxygen is removed, the volume is 10 liters. After the hydrogen is removed, 6 liters of gas remain. The mixture contained:
1. _________ liters of oxygen
2. __________ liters of hydrogen
3. __________ liters of nitrogen
Answer: VT = Voxygen + Vhydrogen + Vnitrogen
1. 2 liters of oxygen (12 − 10 = 2)
2. 4 liters of hydrogen (10 − 6 = 4)
3. 6 liters of nitrogen (left after removal of oxygen and hydrogen)
![]() Determine the mole fractions of each gas in the mixture in frame 44 and express them in decimal form. (Where necessary, round to the nearest thousandth.)
Determine the mole fractions of each gas in the mixture in frame 44 and express them in decimal form. (Where necessary, round to the nearest thousandth.)
1. Mole fraction of O2 = _____________
2. Mole fraction of H2 = _____________
3. Mole fraction of N2 = ____________
Answer:
1. 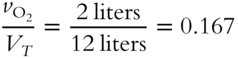
2. 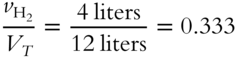
3. 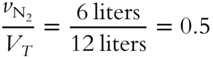
![]() If the total pressure is 1 standard atmosphere (760 torr), what are the partial pressures of each gas in the mixture in frame 45?
If the total pressure is 1 standard atmosphere (760 torr), what are the partial pressures of each gas in the mixture in frame 45?
1. Partial pressure for O2 gas = __________ torr
2. Partial pressure for H2 gas = __________ torr
3. Partial pressure for N2 gas = __________ torr
Answer: Pgas = mole fraction × PT
1. 127 (0.167 × 760 = 127 torr)
2. 253 (0.333 × 760 = 253 torr)
3. 380 (0.5 × 760 = 380 torr)
![]() Let's review. A mixture of N2 gas, NO gas, and NO2 gas was analyzed by the selective absorption of nitrogen oxides. The initial volume of the sample is 300 mL. After treatment with water to remove NO2, the volume is 220 mL. An iron(II) sulfate solution is used to remove NO, after which the volume left is 27 mL. Ignoring water vapor, determine the volume of each gas in the original mixture. The pressure remained constant at 1 standard atmosphere.
Let's review. A mixture of N2 gas, NO gas, and NO2 gas was analyzed by the selective absorption of nitrogen oxides. The initial volume of the sample is 300 mL. After treatment with water to remove NO2, the volume is 220 mL. An iron(II) sulfate solution is used to remove NO, after which the volume left is 27 mL. Ignoring water vapor, determine the volume of each gas in the original mixture. The pressure remained constant at 1 standard atmosphere.
1. ![]() = _________
= _________
2. ![]() = _________
= _________
3. ![]() = _________
= _________
Answer: ![]()
1. ![]()
2. VNO = 193 mL (220 − 27 = 193 mL)
3. ![]() (left over after removal of NO2 and NO)
(left over after removal of NO2 and NO)
![]() What is the mole fraction (to the nearest thousandth) of each gas in the mixture described in frame 47?
What is the mole fraction (to the nearest thousandth) of each gas in the mixture described in frame 47?
1. Mole fraction of NO2 = ________
2. Mole fraction of NO = ________
3. Mole fraction of N2 = ________
Answer:
1. 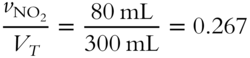
2. 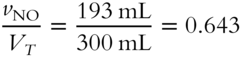
3. 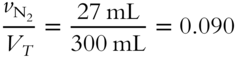
![]() Determine the volume percentage of the gas mixture in frames 47 and 48.
Determine the volume percentage of the gas mixture in frames 47 and 48.
1. Volume percentage of NO2 = ___________
2. Volume percentage of NO = ____________
3. Volume percentage of N2 = ___________
Answer: Volume percentage = mole fraction × 100%
1. Volume percentage of NO2 = 0.267 × 100 % = 26.7%
2. Volume percentage of NO = 0.643 × 100 % = 64.3%
3. Volume percentage of N2 = 0.090 × 100 % = 9.0%
![]() The total pressure of the mixture frames 47 and 48 prior to analysis was 1 standard atmosphere. Determine the partial pressure (to the nearest tenth) of each gas in the mixture.
The total pressure of the mixture frames 47 and 48 prior to analysis was 1 standard atmosphere. Determine the partial pressure (to the nearest tenth) of each gas in the mixture.
1. PT = ___________torr
2. ![]()
3. PNO = ___________torr
4. ![]()
Answer:
1. PT = 760 torr (1 standard atmosphere = 760 torr)
2. ![]()
3. PNO = PT × 0.643 = 760 torr × 0.643 = 488.7 torr
4. ![]()
You have just learned to determine the volumes, partial pressures, and mole fractions of individual gases within a gas mixture. In the next section you will learn how to determine the formula weight of an unknown gas.
GRAHAM'S LAW
![]() Earlier in this chapter, we mentioned that gases readily diffuse (mix spontaneously throughout each other). Even with no breeze blowing the smell of ammonia (a gas) or the scent of a surprised skunk (gaseous vapor) rapidly diffuses through the air. While diffusion denotes the mixing of gases, effusion is the process of a gas going through a small opening into a vacuum. (A vacuum is the absence of a solid, liquid, or gas.)
Earlier in this chapter, we mentioned that gases readily diffuse (mix spontaneously throughout each other). Even with no breeze blowing the smell of ammonia (a gas) or the scent of a surprised skunk (gaseous vapor) rapidly diffuses through the air. While diffusion denotes the mixing of gases, effusion is the process of a gas going through a small opening into a vacuum. (A vacuum is the absence of a solid, liquid, or gas.)
Diffusion indicates one gas mixing into another gas. Effusion indicates a gas moving into a vacuum. A scientist named Graham noted a relationship between the formula weight of a gas and the velocity or speed with which it effuses. Such a relationship is very useful for determining the formula weights of unknown gases. It is also useful in the separation of two gases of different formula weights. Although Graham's Law predicts accurately the velocity of a gas during effusion, the same law can be used as a reasonable approximation of the velocity of a gas during diffusion.
According to Graham's Law, at a constant temperature, the rate (velocity) of effusion of a gas is inversely proportional to the square root of its formula weight. In the equation, a gas of known molecular weight is compared to an unknown gas. In all calculations with Graham's Law, it is assumed that the temperature and pressure are the same for both gases:
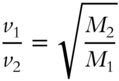

Here is a practical example using Graham's Law. Let oxygen gas (O2) be the known gas. Oxygen has an effusion rate ![]() of 10 milliliters (mL) per second and a formula weight of 32 amu. An unknown gas has an effusion rate (vX) of 5 mL per second. Determine the formula weight (MX) of the unknown gas. (Note that the equation can be modified as follows.)
of 10 milliliters (mL) per second and a formula weight of 32 amu. An unknown gas has an effusion rate (vX) of 5 mL per second. Determine the formula weight (MX) of the unknown gas. (Note that the equation can be modified as follows.)
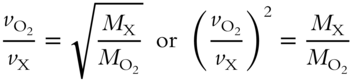
Answer:
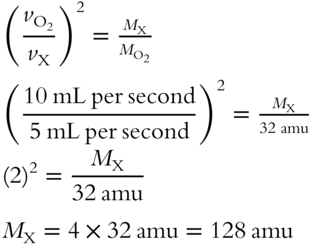
![]() In another situation involving the use of Graham's Law, helium gas (He) has a fairly high velocity of effusion, 50 mL per second, and has a formula weight of 4 amu. An unknown gas has a slower velocity of effusion, 10 mL per second. Using the appropriate equation from the previous frame, determine the formula weight of the unknown gas.
In another situation involving the use of Graham's Law, helium gas (He) has a fairly high velocity of effusion, 50 mL per second, and has a formula weight of 4 amu. An unknown gas has a slower velocity of effusion, 10 mL per second. Using the appropriate equation from the previous frame, determine the formula weight of the unknown gas.
Answer:
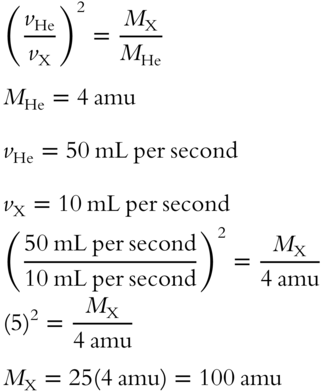
![]() In a different situation, hydrogen (H2) has an effusion rate of 20 mL per second and its formula weight is 2 amu. Oxygen (O2) has a formula weight of 32 amu.
In a different situation, hydrogen (H2) has an effusion rate of 20 mL per second and its formula weight is 2 amu. Oxygen (O2) has a formula weight of 32 amu.
1. What is the expected effusion rate of O2 in this situation? _____________
2. Oxygen, with a formula weight of 32 amu, is a heavier gas than hydrogen. Which gas has the greater effusion rate, the heavier oxygen or the lighter hydrogen? ________________
Answer:
1. Note that either of these equations is correct. We will use the equation on the right for convenience in the calculation.
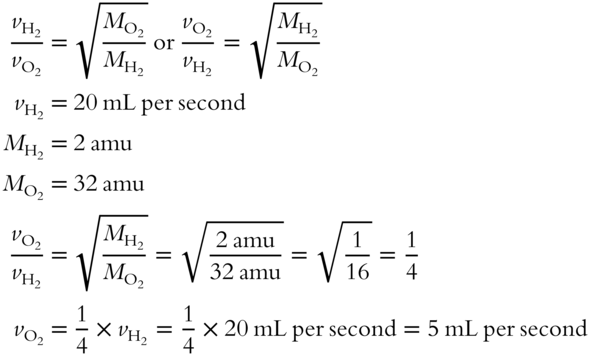
2. The hydrogen gas has an effusion rate of 20 mL per second and oxygen gas has an effusion rate of only 5 mL per second. The lighter hydrogen has the greater rate of effusion (four times greater than oxygen).
![]() The heavier the formula weight of a gas, the slower the effusion rate, according to Graham's Law. Arrange the four gases listed below in order from the slowest to the fastest effusion rate.
The heavier the formula weight of a gas, the slower the effusion rate, according to Graham's Law. Arrange the four gases listed below in order from the slowest to the fastest effusion rate.
· CO2 has a formula weight of 44.
· N2 has a formula weight of 28.
· F2 has a formula weight of 38.
· O2 has a formula weight of 32.
Slowest _____, _____, _____, _____, Fastest
Answer: CO2, F2, O2, N2
![]() The equation for Graham's Law can be modified to deal with the density of gases. The relationship between density and effusion rates will be dealt with in a few frames. First, let's clarify the meaning of density as applied to gases. Density is a measure of weight per unit volume. For gases, we will use grams per liter as the measure of density.
The equation for Graham's Law can be modified to deal with the density of gases. The relationship between density and effusion rates will be dealt with in a few frames. First, let's clarify the meaning of density as applied to gases. Density is a measure of weight per unit volume. For gases, we will use grams per liter as the measure of density.
You have already learned that the volume of a gas is dependent on pressure and temperature. To have an accurate measure of the density of a gas, we must specify a temperature and pressure. Standard temperature and pressure (abbreviated STP) have been set arbitrarily at 0°Celsius and 1 atm. At STP, 1 mol of any gas occupies 22.4 liters. Note that we are still treating every gas as a theoretically ideal gas. The volume of 22.4 liters per mole at STP is a reasonable approximation for real gases.
Assuming that O2 gas behaves ideally, 1 mol of O2 at STP occupies what volume? _________________
Answer: 22.4 liters
![]() Unless otherwise stated we will assume that all gases behave ideally. Since oxygen (O2) has a volume of 22.4 liters per mole at STP, we can calculate its density. The formula weight (the weight of 1 mol) of O2 is 32.0 grams. The density of O2 at STP to the nearest hundredth of a gram per liter is:
Unless otherwise stated we will assume that all gases behave ideally. Since oxygen (O2) has a volume of 22.4 liters per mole at STP, we can calculate its density. The formula weight (the weight of 1 mol) of O2 is 32.0 grams. The density of O2 at STP to the nearest hundredth of a gram per liter is:
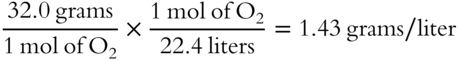
Calculate the density of N2 at STP. __________________________________
Answer: Using the periodic table, the formula weight of N2 is 28.0 grams (2 × 14.0 grams)
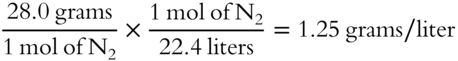
(Remember to express the density in grams per liter.)
![]() What is the density of Cl2 at STP?___________________________________
What is the density of Cl2 at STP?___________________________________
Answer: 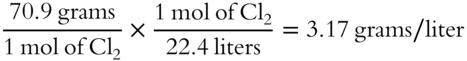
![]() An unknown gas was found to have a formula weight of 128.0 grams. What is its density at STP?____________________
An unknown gas was found to have a formula weight of 128.0 grams. What is its density at STP?____________________
Answer: 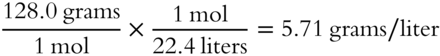
![]() The equation for Graham's Law can be rewritten to include densities:
The equation for Graham's Law can be rewritten to include densities:
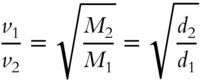
By eliminating the part that includes formula weights, we have:
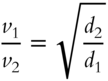
In the equation, d1 indicates the density of gas 1 and d2 indicates the density of gas 2. Here is an example using this formula. A known gas (oxygen) has a density ![]() of 1.43 grams per liter and an effusion rate of 10.0 mL per second. An unknown gas (X) has an effusion rate of 5.0 mL per second. The density of the unknown gas can be determined as follows:
of 1.43 grams per liter and an effusion rate of 10.0 mL per second. An unknown gas (X) has an effusion rate of 5.0 mL per second. The density of the unknown gas can be determined as follows:
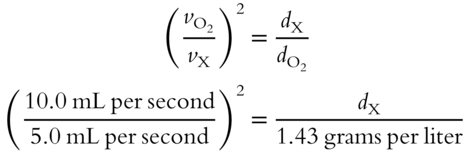
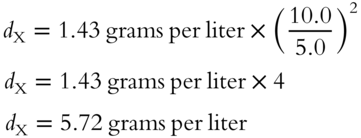
Another unknown gas has an effusion rate of 8.0 mL per second. Using oxygen gas as a reference (effusion rate and density are the same as in the previous example), determine the density of the unknown gas. __________
Answer:
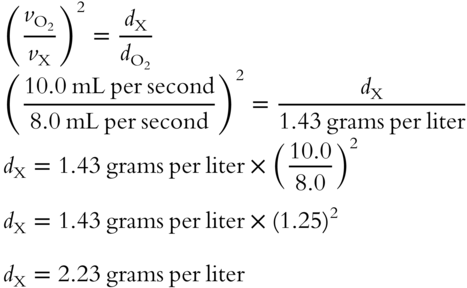
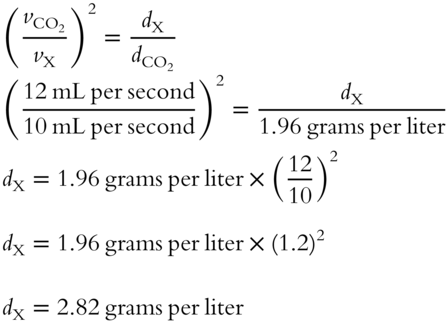
![]() Carbon dioxide (CO2) is used as a reference gas with a density of 1.96 grams per liter and an effusion rate of 12.0 mL per second. An unknown gas has an effusion rate of 10.0 mL per second. Determine the density of the unknown gas. __________
Carbon dioxide (CO2) is used as a reference gas with a density of 1.96 grams per liter and an effusion rate of 12.0 mL per second. An unknown gas has an effusion rate of 10.0 mL per second. Determine the density of the unknown gas. __________
Answer:
![]() Once you have found the density of an unknown gas, it is a simple matter to determine its formula weight. Just reverse the procedure used in finding a density from formula weight. For example, the unknown gas in frame 59 had a density of 2.23 grams per liter. Assume STP.
Once you have found the density of an unknown gas, it is a simple matter to determine its formula weight. Just reverse the procedure used in finding a density from formula weight. For example, the unknown gas in frame 59 had a density of 2.23 grams per liter. Assume STP.
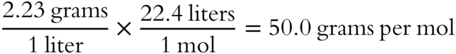
In frame 60, you determined that an unknown gas had a density of 2.82 grams per liter. Determine its formula weight to the nearest tenth of a gram per mole. Assume STP.______________
Answer: 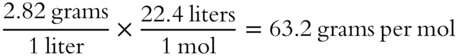
THE COMBINED GAS LAW
![]() Earlier in this chapter, you learned about Boyle's Law and Charles's Law. We will now combine these two laws. To review, Boyle's Law states that PV = constant (at constant temperature). A practical expression of Boyle's Law is P1V1 = P2V2 at constant temperature. Charles's Law states
Earlier in this chapter, you learned about Boyle's Law and Charles's Law. We will now combine these two laws. To review, Boyle's Law states that PV = constant (at constant temperature). A practical expression of Boyle's Law is P1V1 = P2V2 at constant temperature. Charles's Law states
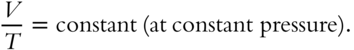
A practical expression of Charles's Law is
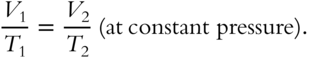
Boyle's and Charles's Laws can be combined into
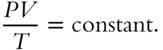
A practical expression of the combined gas law is
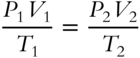
In using the combined gas law or any gas law involving temperature, what temperature scale must be used?__________________
Answer: Absolute or Kelvin or K (Any of these answers is acceptable.)
![]() We can modify the combined gas law as follows.
We can modify the combined gas law as follows.
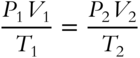
Multiplying both sides by T2 results in 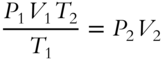 .
.
Dividing both sides by P2 results in 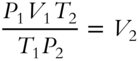 .
.
A sample of helium occupies 1000 liters (V1) at 15°C (T1) and 763 torr (P1). The temperature is changed to −6°C and the pressure to 420 torr. Use the combined gas law from frame 62 to find the new volume of the gas (V2) to the nearest liter. _________________________
Answer: Change all temperatures to Kelvin scale (add 273 to Celsius scale).
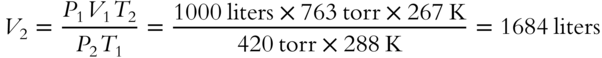
![]() A sample of oxygen gas occupies 400 mL at 20°C and 740 torr. If the conditions are changed so that the gas is warmed to 30°C and occupies 425 mL, what is its new pressure to the nearest torr?_______________
A sample of oxygen gas occupies 400 mL at 20°C and 740 torr. If the conditions are changed so that the gas is warmed to 30°C and occupies 425 mL, what is its new pressure to the nearest torr?_______________
Answer: 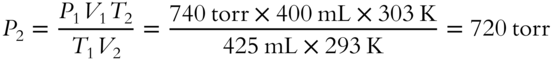
THE IDEAL GAS LAW EQUATION
![]() In using the combined gas law, we have used an equation with six items, five of which are given and one of which is unknown. A simpler equation can be made from the combined gas law by including a constant to represent gases at STP:
In using the combined gas law, we have used an equation with six items, five of which are given and one of which is unknown. A simpler equation can be made from the combined gas law by including a constant to represent gases at STP:
![]()
In this equation, P, V, and T describe the existing gas conditions. The symbol n represents the number of moles of the gas. The symbol R represents the universal gas constant. This equation is commonly known as the ideal gas law equation. Rewrite the ideal gas law equation so that only R remains on one side of the equation.
R = _______________
Answer: ![]() (divide both sides by nT)
(divide both sides by nT)
![]() The universal gas constant (R) is a numerical value made up of the combination of standard pressure (1 atm or 760 torr), standard temperature (273 K), and the fact that 1 mol of a gas occupies a volume of 22.4 liters at STP.
The universal gas constant (R) is a numerical value made up of the combination of standard pressure (1 atm or 760 torr), standard temperature (273 K), and the fact that 1 mol of a gas occupies a volume of 22.4 liters at STP.
The following value of R is appropriate for calculations dealing with pressure expressed in atmospheres, volume expressed in liters, temperature expressed in Kelvin degrees, and the quantity of gas expressed in moles:
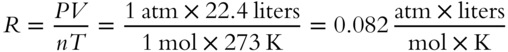
We can also calculate a value of R for use in problems dealing with pressure expressed in torr, volume in liters, temperature in Kelvin, and quantity in moles:
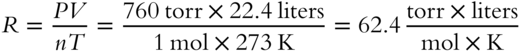
If volume is expressed in milliliters, pressure in torr, temperature in Kelvin, and quantity in moles, we have the following value of R:
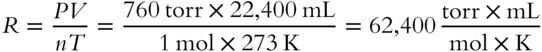
The appropriate value of R must be selected based on the units of P and V.
· If V is expressed in liters and P in torr, then R is 62.4.
· If V is expressed in liters and P in atmospheres, then R is 0.082.
· If V is expressed in milliliters and P in torr, then R is 62,400.
It is assumed the temperature is expressed in Kelvin and n is expressed in moles. Here is an example using the ideal gas law equation. Half a mole of gas (n) has a pressure of 2 atm (P) and a temperature (T) of 341 K. We want to calculate its volume in liters using the ideal gas equation. Since V is expressed in liters and P in atmospheres, R must be
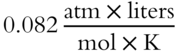
Dividing both sides of the equation PV = nRT by P results in

In another example, 2 mol of a gas occupies a volume of 4 liters when the temperature is 300 K. What is the pressure (expressed in torr) of the gas?_____________
Answer: Since V is expressed in liters and P in torr, R must be 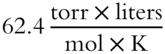 .
.
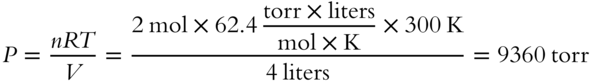
![]() Three moles of a gas are compressed to 10,000 torr. The temperature is 27°C. What is the volume of the gas in milliliters?___________________________
Three moles of a gas are compressed to 10,000 torr. The temperature is 27°C. What is the volume of the gas in milliliters?___________________________
Answer:
Since V is expressed in milliliters and P in torr, R must be 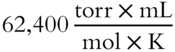 .
.
Celsius temperature must be changed to Kelvin (27 + 273 = 300 K).
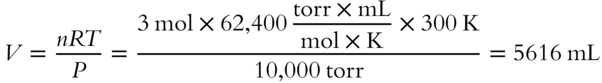
![]() If 1.5 mol of gas occupies 41 liters at a pressure of 3 atm, what is the temperature of the gas?___________
If 1.5 mol of gas occupies 41 liters at a pressure of 3 atm, what is the temperature of the gas?___________
Answer: Since V is expressed in liters and P in atmospheres, R must be
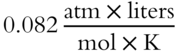
Divide both sides of the equation by nR:
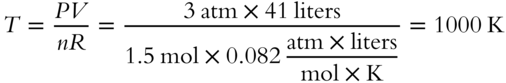
![]() We can also use the ideal gas equation to determine the formula weights of gases. The formula weight of neon gas (Ne) is 20.18 grams per mol. A sample of Ne gas weighing 201.8 grams represents the weight of how many moles of Ne gas?__________________
We can also use the ideal gas equation to determine the formula weights of gases. The formula weight of neon gas (Ne) is 20.18 grams per mol. A sample of Ne gas weighing 201.8 grams represents the weight of how many moles of Ne gas?__________________
Answer: 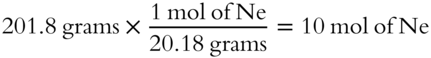
![]() In the equation PV = nRT, the symbol n represents the number of moles of a gas. The number of moles of a gas is equal to the following, where M is the formula weight of the gas in grams per mole:
In the equation PV = nRT, the symbol n represents the number of moles of a gas. The number of moles of a gas is equal to the following, where M is the formula weight of the gas in grams per mole:
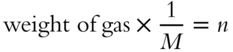
The equation PV = nRT can be modified to include the above equation for the number of moles. Show this below.
PV = _____________________
Answer: 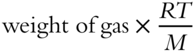
![]() The equation you just wrote can be solved for formula weight:
The equation you just wrote can be solved for formula weight:
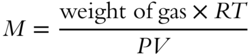
Determine the formula weight of a gas if 20 grams of the gas occupies a volume of 6 liters at a pressure of 2 atm and a temperature of 300 K. Express in grams per mole. ________________
Answer: Since V is in liters and P is in atmospheres, R must be 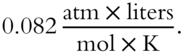
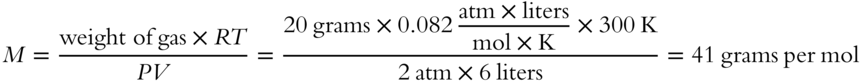
![]() A sample of gas weighing 1.00 grams has a volume of 500 mL at a temperature of 77 °C and a pressure of 546 torr. What is the formula weight of the gas?__________
A sample of gas weighing 1.00 grams has a volume of 500 mL at a temperature of 77 °C and a pressure of 546 torr. What is the formula weight of the gas?__________
Answer: Since V is in milliliters and P is in torr, R must be
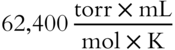
Change 77°C to Kelvin (77 + 273 = 350 K).
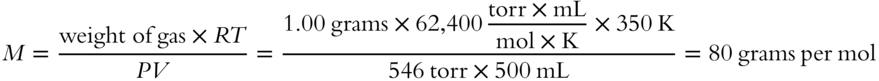
![]() All of the gas law equations thus far have dealt with theoretically ideal gases. The real gases in the world behave ideally at high temperatures and at low pressures. However, at very high pressures and low temperatures, real gases deviate from the ideal.
All of the gas law equations thus far have dealt with theoretically ideal gases. The real gases in the world behave ideally at high temperatures and at low pressures. However, at very high pressures and low temperatures, real gases deviate from the ideal.
In real gases at high pressures, the volume of the gas molecules actually makes up a significant part of the gas volume. This causes the real gas to be less compressible than predicted by the ideal gas law.
At very low temperatures, the gas molecules attract one another and the real gas becomes more compressible than predicted by the ideal gas law. These attractive forces between the molecules are known as van der Waals forces.
1. In the kinetic molecular theory, what assumption is made about the volume of the molecules of an ideal gas?_________________
2. The attraction of gas molecules for each other becomes significant at which range of temperature, very high or very low?________________
Answer: (a) that the molecules of an ideal gas occupy no volume; (b) very low
At sufficiently low temperatures, the van der Waals forces become strong enough to overcome the kinetic energy of the gas molecules. The result is that gas molecules stick together and form small droplets. These droplets combine to form a liquid. You will learn more about van der Waals forces in Chapter 10 on liquids.
Additional constants have been determined to add to the ideal gas law equation that make the equation more correct for the effects of gas molecule volume at high pressures and van der Waals forces at low temperatures. This equation, known as the van der Waals's equation of state for a real gas, is beyond the scope of this book.
Now you know that gases may be compressed (as in a bicycle pump), exert pressure on the walls of their containers (as in a tire or balloon), have different densities (air versus helium), and effuse or diffuse so you can smell them.
You also know that all gases behave in a similar manner and are affected by changes in temperature and pressure. The laws presented in this chapter apply only to the gaseous state. The remaining states of matter, liquids, and solids behave quite differently than gases. The next two chapters discuss the solid and liquid states. In contrast to gases, solids and liquids do not all behave in a similar manner.
SELF-TEST
This self-test is designed to show how well you have mastered this chapter's objectives. Correct answers and review instructions follow the test. Round answers to the nearest tenth.
1. Which of the following assumptions about the kinetic molecular theory of gases is incorrect?
1. Gases are made up of individual molecules that are in constant rapid motion.
2. Gas molecules are closer together than molecules of a liquid or solid.
3. Gas molecules possess kinetic energy that varies with temperature.
4. Gas molecules occupy no volume and therefore have no attraction for each other.
2. 15.5 Liters of neon gas at 1 atm of pressure is compressed to 4.4 liters of volume. What is the pressure at this new volume?
3. 2.75 Liters of nitrogen gas at 543 torr is compressed to a pressure of 965 torr. What is the resulting volume at this new pressure?
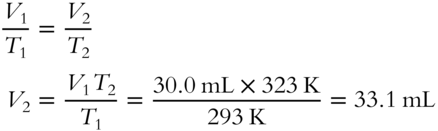
4. A sample of Cl2 gas occupies a volume of 30.0 mL at 20°C. What volume would it occupy at 50°C? Assume the pressure remains constant and use Charles's Law.________________________________________________
5. A sample of oxygen gas occupying a volume of 325.0 mL at 30oC is heated to 65oC. Calculate the final volume of this gas.
6. A sample of helium gas occupying a volume of 250.0 mL at constant pressure at 100K is heated and expands to a volume of 850.0 mL. Calculate the final temperature of the gas at these conditions.
7. 5.00 mol H2 at 1.10 atm of pressure and 308 K occupies a volume of ______ L.
8. 8.55 × 10-2 mol N2 at 740 torr of pressure and 298 K occupies a volume of ______ L (answer to the nearest hundredth).
9. A sample of CO2 gas occupies 350 mL at 27°C and 790 torr. What pressure would it exert if it occupied 175 mL and the temperature was changed to 57°C? Use the combined gas law equation._________________________
10. What volume would 22.0 grams of N2O occupy at STP? _______________
11. What is the density of fluorine (F2) at STP? _________________________
12. Arrange the following gases in the order of increasing effusion rates. CO, O2, SO2, NH3, F2 _____, ______, _______, _______, _______
13. A mixture of CO2 and O2 is used to “decompress” deep sea divers to prevent the “bends.” If the mixture is 70% O2 and 30% CO2 by volume, what is:
1. the volume of O2 present in 50.0 liters of the mixture?_____________
2. the mole fraction of O2 in the mixture?_________________________
3. the pressure exerted by the O2 if the pressure of the mixture is 1,000 torr?________________________________________________
14. Which of the following can account for the fact that you can smell a skunk even though you may not see it (more than one is correct)?
o ____ (a) Gases are in rapid random motion.
o ____ (b) Gases may be compressed.
o ____ (c) Gas molecules are far apart.
o ____ (d) Gas volumes are directly proportional to the absolute temperature.
o ____ (e) Gas volumes are inversely proportional to the pressure.
15. Calculate the formula weight of 1.68 grams of a gas that occupies 1.35 liters at STP using the ideal gas law equation.
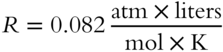
ANSWERS
Compare your answers to the self-test with those given below. If you answered all questions correctly, you are ready to proceed to the next chapter. If you got any wrong, review the frames indicated in parentheses following the answers. If you got several questions wrong, you should probably reread the chapter carefully.
1. (b.) is incorrect [frames 1-9]
2. 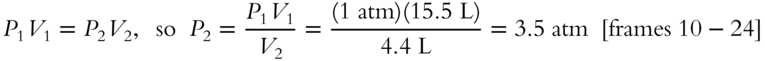
3. 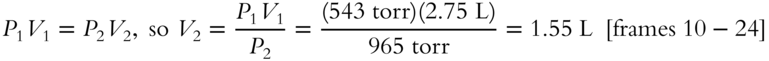
4.
(frames 25—31)
5. 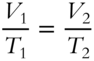 , so
, so 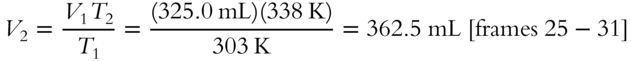
6. 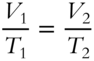 , so
, so 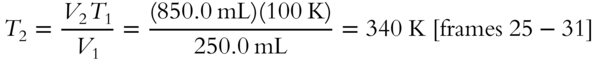
7. PV = nRT, so 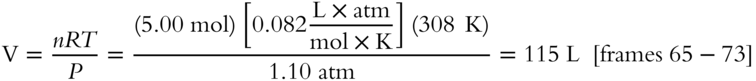
8. PV = nRT, so 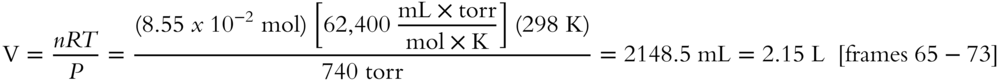
9.
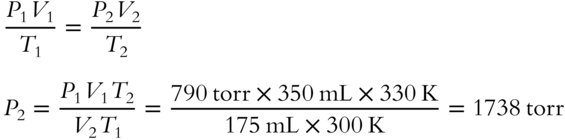
(frames 62—64)
10.
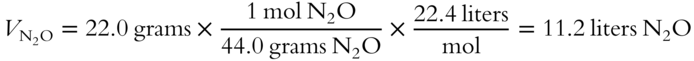
(frame 55)
11.
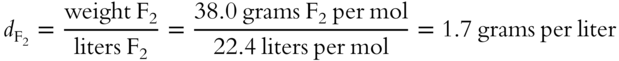
(frames 56—58)
12.
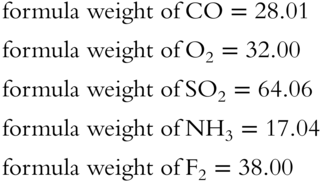
Therefore, increasing effusion rate from left to right is:
SO2, F2, O2, CO, NH3 (frames 51—54)
13.
1. ![]() (frames 39, 40)
(frames 39, 40)
2. 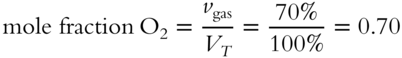
3. ![]()
(frames 36, 37)
14. (a) and (c) (frames 1, 6, 51)
15.
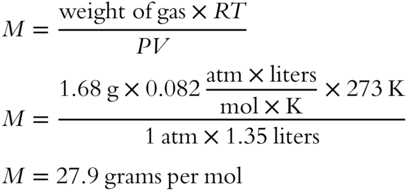
(frames 65—72)
THE USES OF HYDROGEN GAS
The lightest gas we have is made up of two hydrogen atoms (H2), known as elemental hydrogen gas or just hydrogen gas. Not only is it the lightest elemental gas we have, it is also extremely flammable, being able to sustain a fire after it is ignited. Oxygen (O2) is a heavier diatomic gas but it is not as flammable since these substances require O2 to burn.
When the reactants hydrogen gas and oxygen gas mix no, reaction occurs:
![]()
However, the mixture will undergo a chemical reaction with the help of a spark or flame. The results can prove disastrous and the amount of heat released is tremendous even when only moderate quantities of H2 and O2 are present for reaction:
![]()
On 6 May 1937 in Manchester Township in New Jersey, a German passenger airship named the Hindenburg caught fire and was destroyed. This aircraft was filled with hydrogen gas, making use of this light gas for flight (Figure 8.1).

Figure 8.1 Hindenburg disaster. Source: Photo by Sam Shere, now in USA public domain.
At one point, the aircraft sprung a leak of hydrogen gas and it began to mix with the oxygen in the air. The mixture was able to ignite and the Hindenburg was quickly engulfed in flames. The aircraft crashed and 36 people died out of the 96 people onboard. One person on the ground also died.
This was a large-scale chemical reaction where water vapor was produced as the product.
Other reactions that involve hydrogen are the chemical reactions in fuel cells. A fuel cell is a device that converts chemical energy into electrical energy. These cells are similar to batteries but require a continuous source of fuel, most often hydrogen gas (H2) (Figure 8.2).
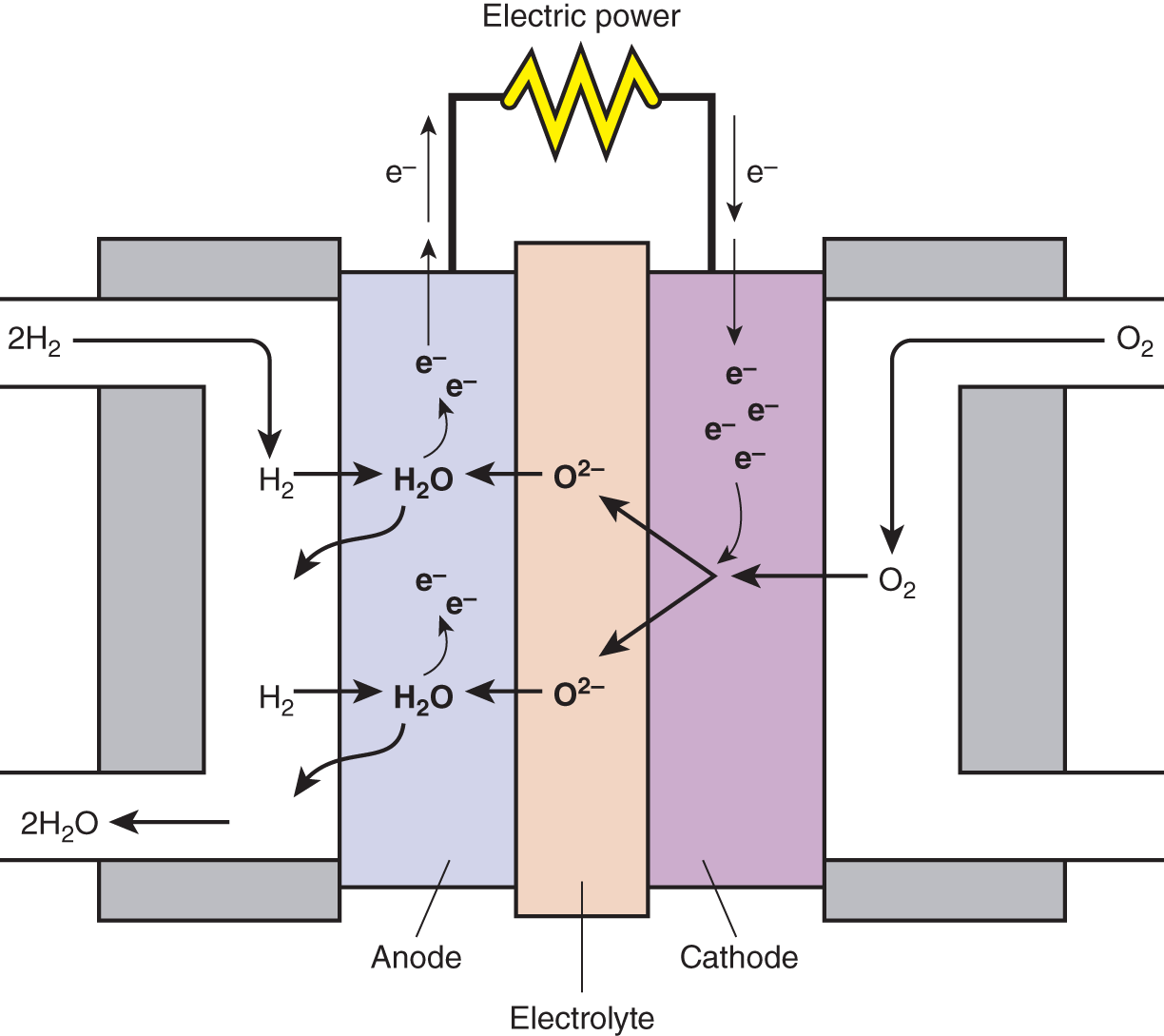
Figure 8.2 Schematic of a hydrogen fuel cell (https://en.wikipedia.org/wiki/File:Solid_oxide_fuel_cell.svg).
The chemical reactions that occur within the fuel cell are:
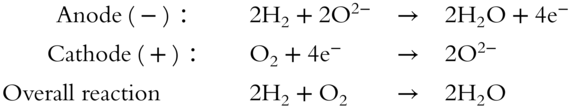
You'll notice that the overall reaction within the fuel cell is exactly the same reaction as the tragedy that occurred in the Hindenburg disaster. Again, hydrogen gas and oxygen gas are the reactants and water is the product. A key difference in the fuel cell and the Hindenburg aircraft is that the fuel cell uses far less hydrogen gas, making them much safer.
Fuel cells are finding applications in diverse ways. For instance, they have been used for automobiles, satellites, and submarines. It's their ability to generate clean energy that clearly sets them apart from the typical automobile engine. In fact, fuel cells offer two advantages that typical automobiles do not possess. First, fuel cells are more efficient than the combustion engine. Their increased efficiency could range from 5% more efficient up to 35% more efficient. Second, their by-product is water, instead of the CO2 and other gases that can be released from cars. In other words, the fuel cell does not contribute to the production of greenhouse gases!
Fuels cells have a lot of potential for the future; however, major hurdles need to be overcome before they are in the general public's automobiles. Their technology makes them quite expensive and they are prone to failure (i.e., they have a lower reliability than the combustion engine).